on ABSCIENCES (EPA:AB)
AB Science Faces Setback in EMA Approval for ALS Treatment
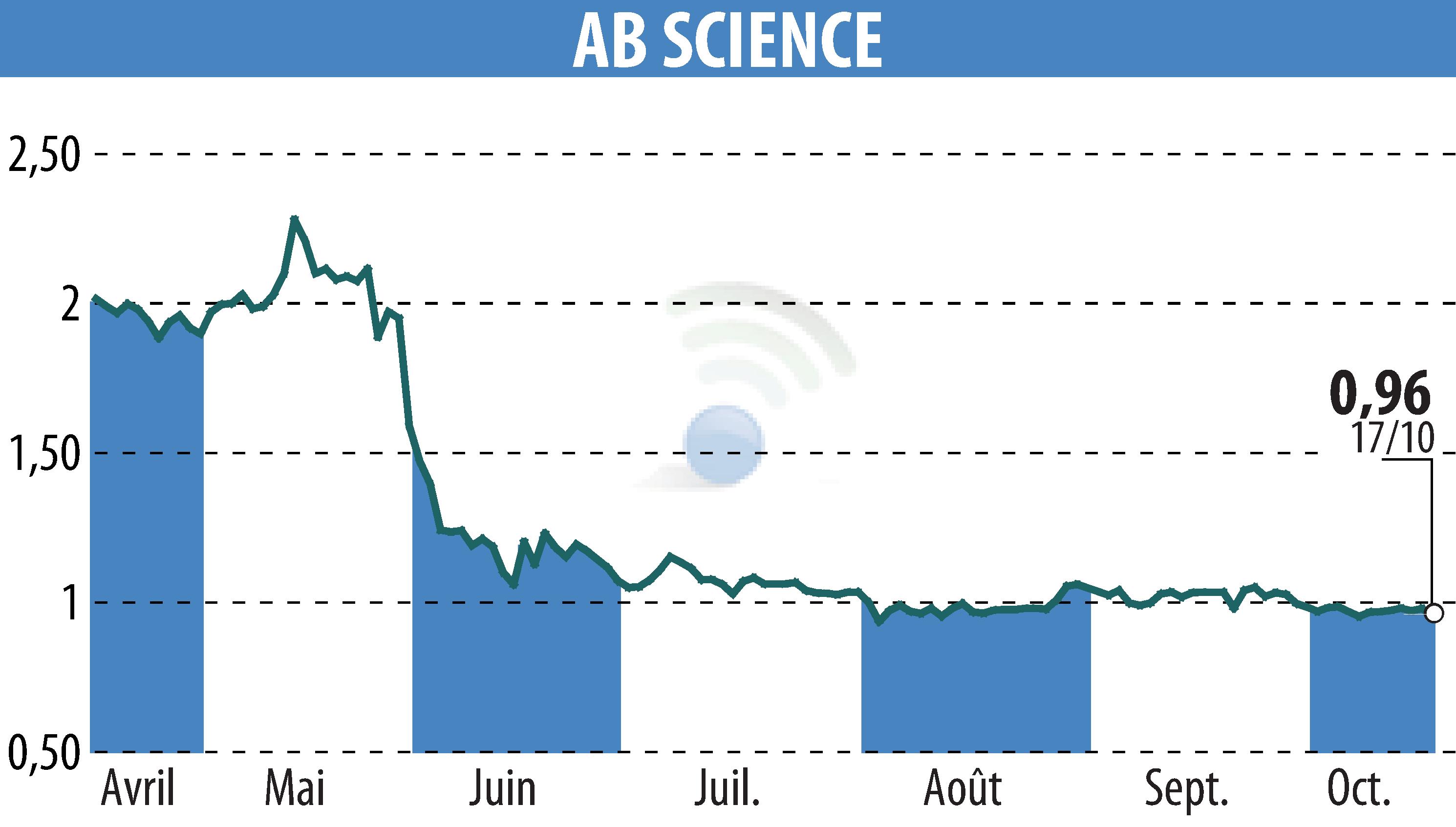
AB Science SA announced that the European Medicines Agency (EMA) has maintained its negative stance on granting conditional marketing authorization for masitinib in treating amyotrophic lateral sclerosis (ALS). The decision was made during the Committee for Medicinal Products for Human Use meeting from October 14 to 17, 2024. This follows AB Science's request for a re-examination after a similar decision in June 2024.
Despite challenges in obtaining conditional approval, AB Science pursued re-examination due to the pressing need for ALS treatment options. A previous study indicated a median survival increase of 6 months in normal progressors and a 12-month improvement in those with no complete function loss. A Scientific Advisory Group supported the study's approach but indicated that the findings alone are insufficient for authorization.
In Canada, AB Science will not pursue a reconsideration after Health Canada identified key analyses as new data. AB Science plans to concentrate on a confirmatory phase 3 program to seek full approval for masitinib in ALS.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABSCIENCES news
