on ABIVAX (EPA:ABVX)
Abivax Reaches Key Milestone with Recruitment of 600 Patients for Phase 3 ABTECT Trial
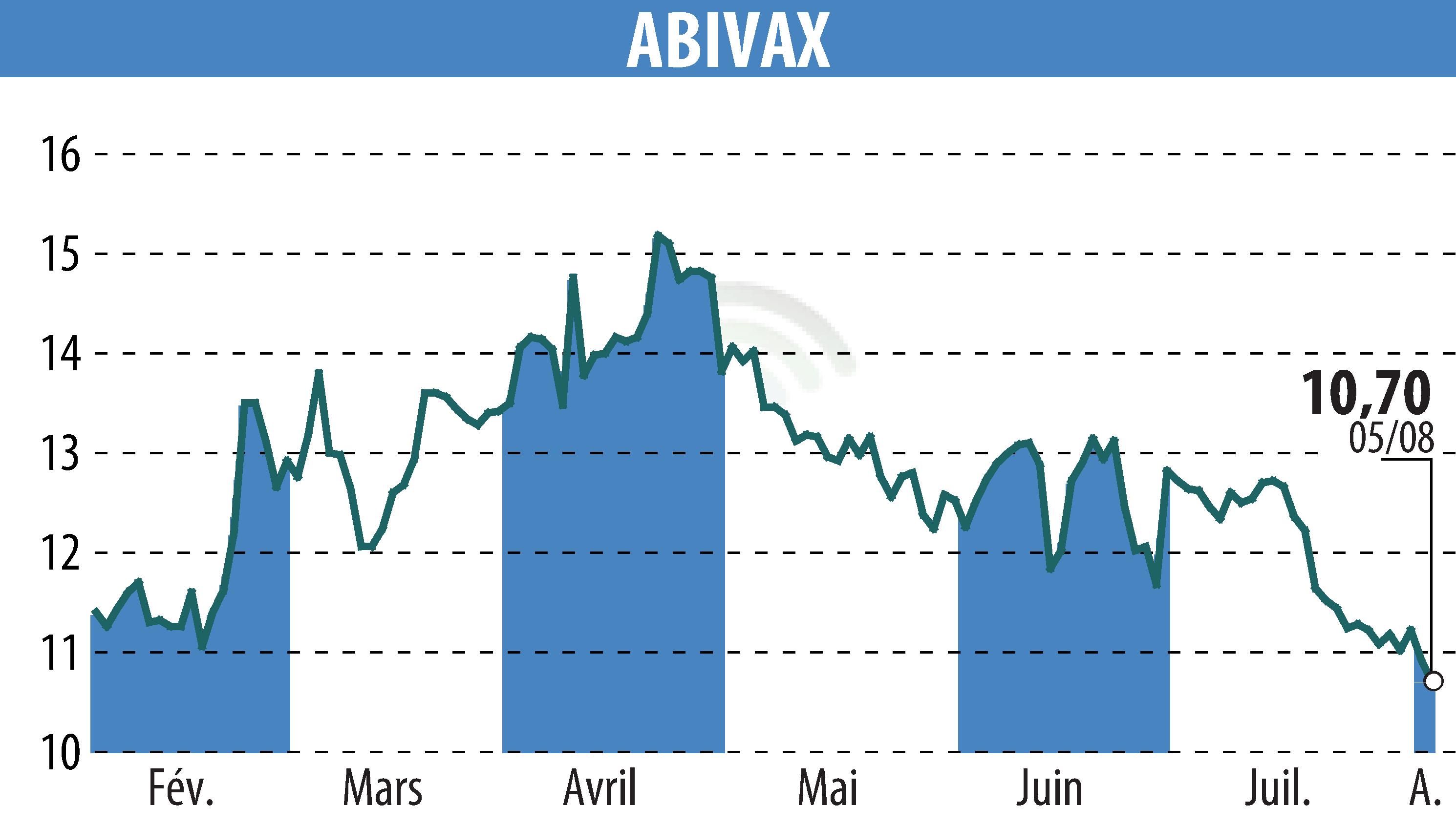
Abivax, a biotechnology company based in Paris, is continuing its phase 3 ABTECT trial of Obefazimod in the treatment of ulcerative colitis (UC). In July 2024, the company enrolled its 600th patient, approaching the end of enrollment planned for early 2025. The trial focuses on moderately to severely active UC cases.
The data collected so far shows similarities with the Phase 2b trial results, reinforcing optimism about Abivax's prospects of completing recruitment on time. The company has updated its information on its website to reflect these advancements.
Obefazimod, Abivax's lead drug candidate, has demonstrated promising efficacy in phase 2. The launch of a phase 2b clinical trial for Crohn's disease as well as the evaluation of combination therapies for the treatment of UC are also in progress. course.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABIVAX news
