on ABIVAX (EPA:ABVX)
Abivax recruits first patient for its clinical trial on Crohn's disease
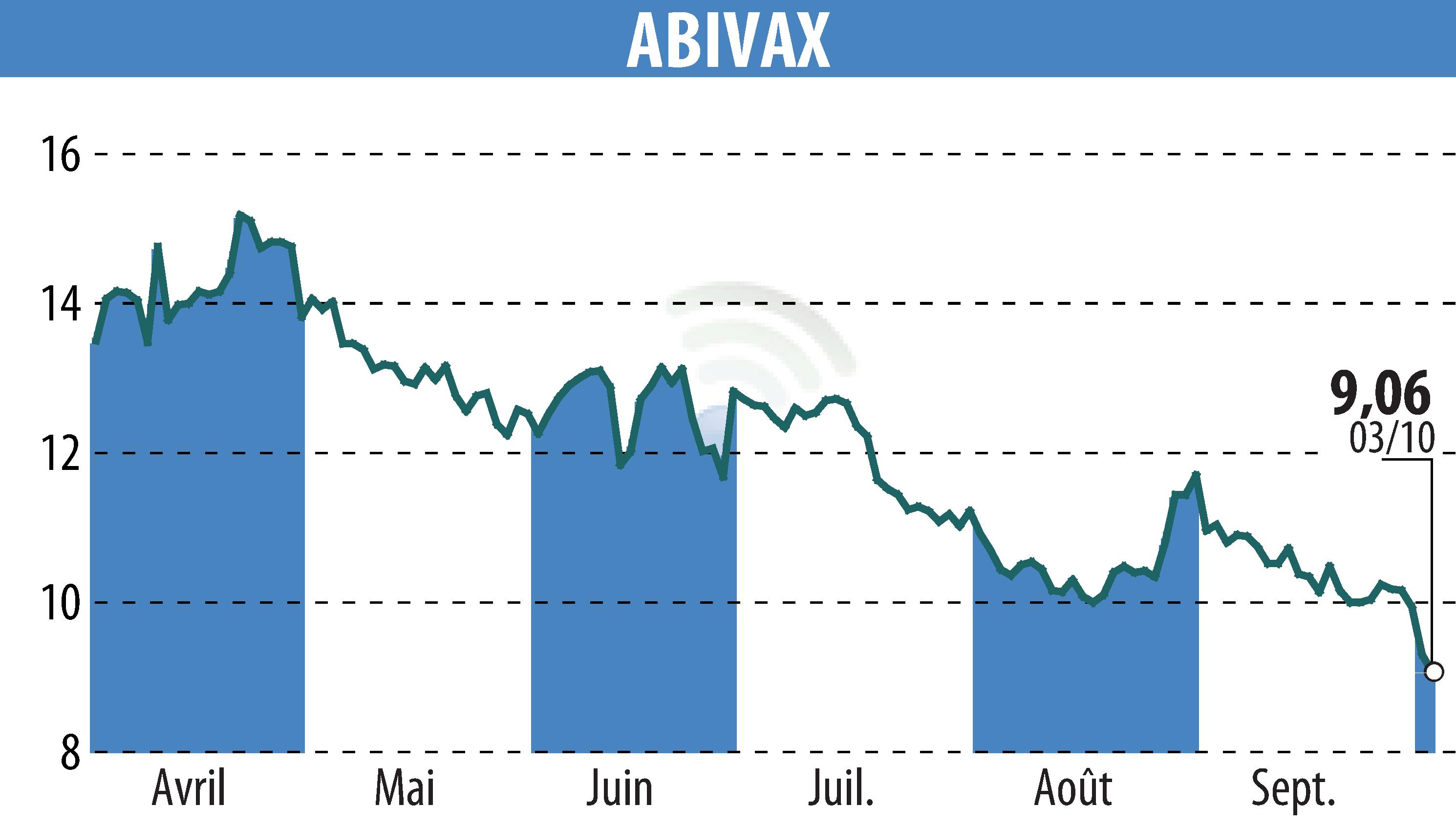
Biotechnology company Abivax announced the enrollment of the first patient in its Phase 2b ENHANCE-CD clinical trial. This trial aims to evaluate the efficacy and safety of the drug obefazimod in patients with Crohn's disease.
Designed as a double-blind, multicenter trial, it includes a 12-week induction phase, a 40-week maintenance phase, and a 48-week extension phase. The primary objective is to analyze the safety and tolerability of obefazimod compared to placebo.
This advance could offer a convenient oral therapeutic option for people with moderately to severely active disease.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABIVAX news
