on BIOMERIEUX (EPA:BIM)
BioMérieux's Diagnostic Test Receives FDA Clearance
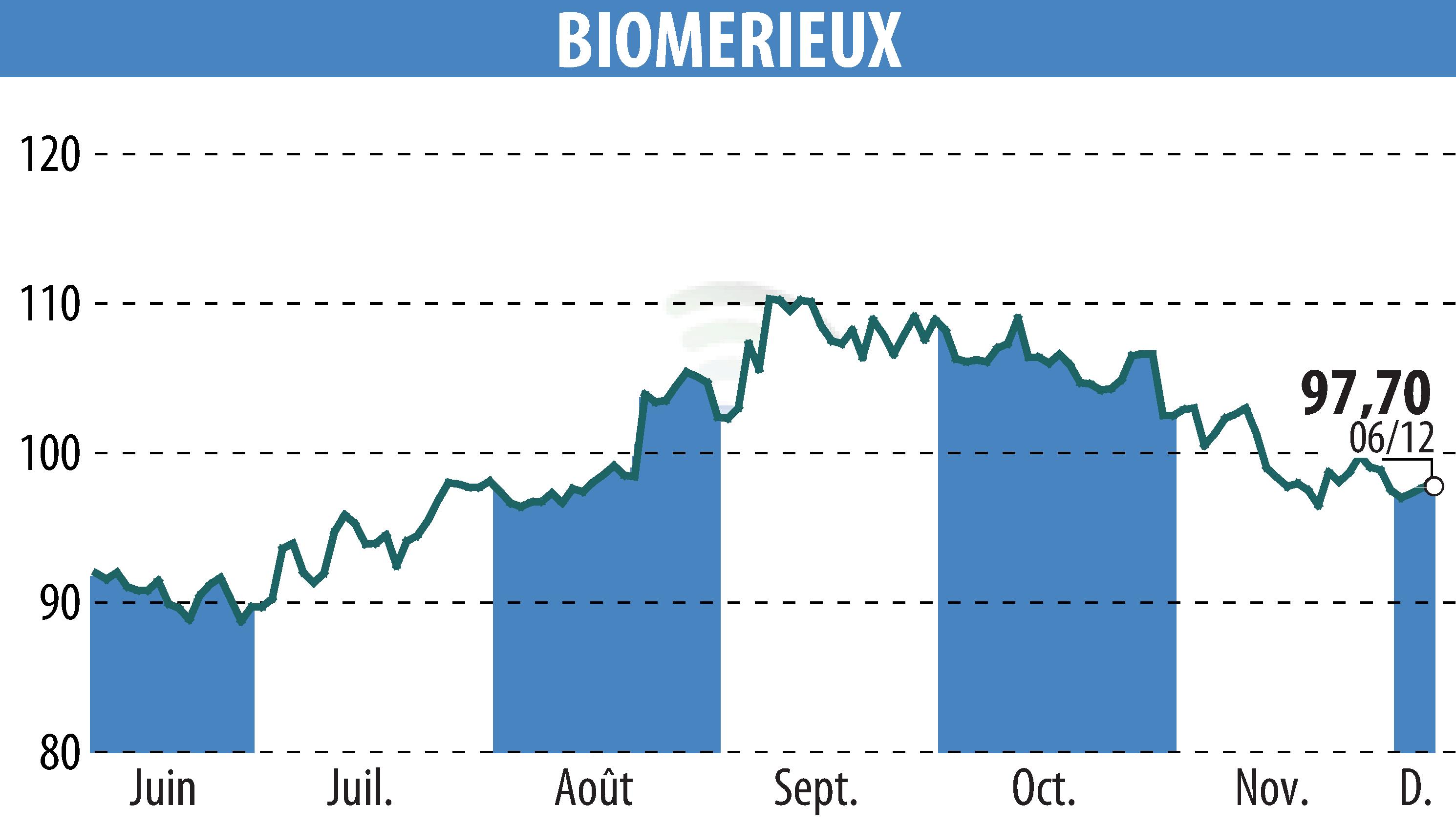
bioMérieux, a leader in in vitro diagnostics, has announced that its BIOFIRE® FILMARRAY® Tropical Fever Panel has received Special 510(k) clearance from the U.S. FDA. This PCR testing solution rapidly identifies pathogens in patients with unexplained fevers, aiding in more effective treatment. Tropical fevers such as malaria and dengue affect millions, and timely diagnosis is crucial.
The panel provides healthcare providers with a tool to quickly detect pathogens common in tropical fevers, potentially leading to better patient management. The test differentiates between Plasmodium species, vital for malaria treatment. It requires minimal preparation time on the BIOFIRE® systems.
bioMérieux plans a commercial launch in early 2025, with plans for global deployment. The development involved collaboration with U.S. agencies, emphasizing the crucial role of public-private partnerships in health innovation.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all BIOMERIEUX news
