on CROSSJECT (EPA:ALCJ)
Crossject Advances ZEPIZURE® Supply Chain Readiness
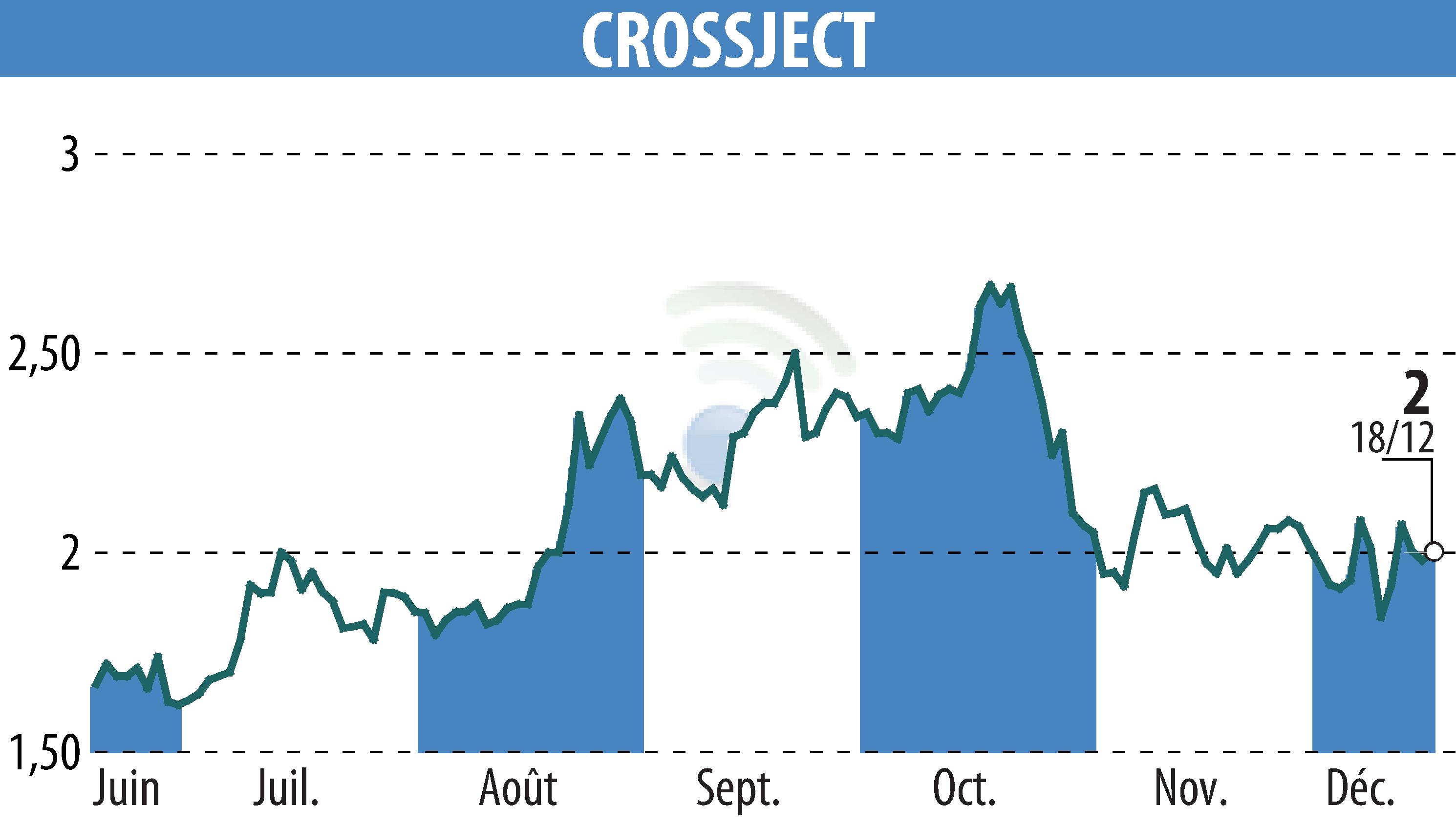
French specialty pharma company Crossject has successfully passed an ISO 13485 audit for its sites in Dijon and Gray. This audit, conducted by the British Standards Institution, confirms the company's compliance with international manufacturing standards. The focus is on the ZEPIZURE® epilepsy rescue therapy, based on the ZENEO® needle-free auto-injector.
CEO Patrick Alexandre stated that the certification aligns their manufacturing sites and processes with global standards, providing a robust foundation for U.S. commercialization. The audit results boost the company's confidence as they increase production and prepare for regulatory approvals and market entry.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all CROSSJECT news
