on CROSSJECT (EPA:ALCJ)
CROSSJECT: Progress in the EUA authorization application for ZEPIZURE®
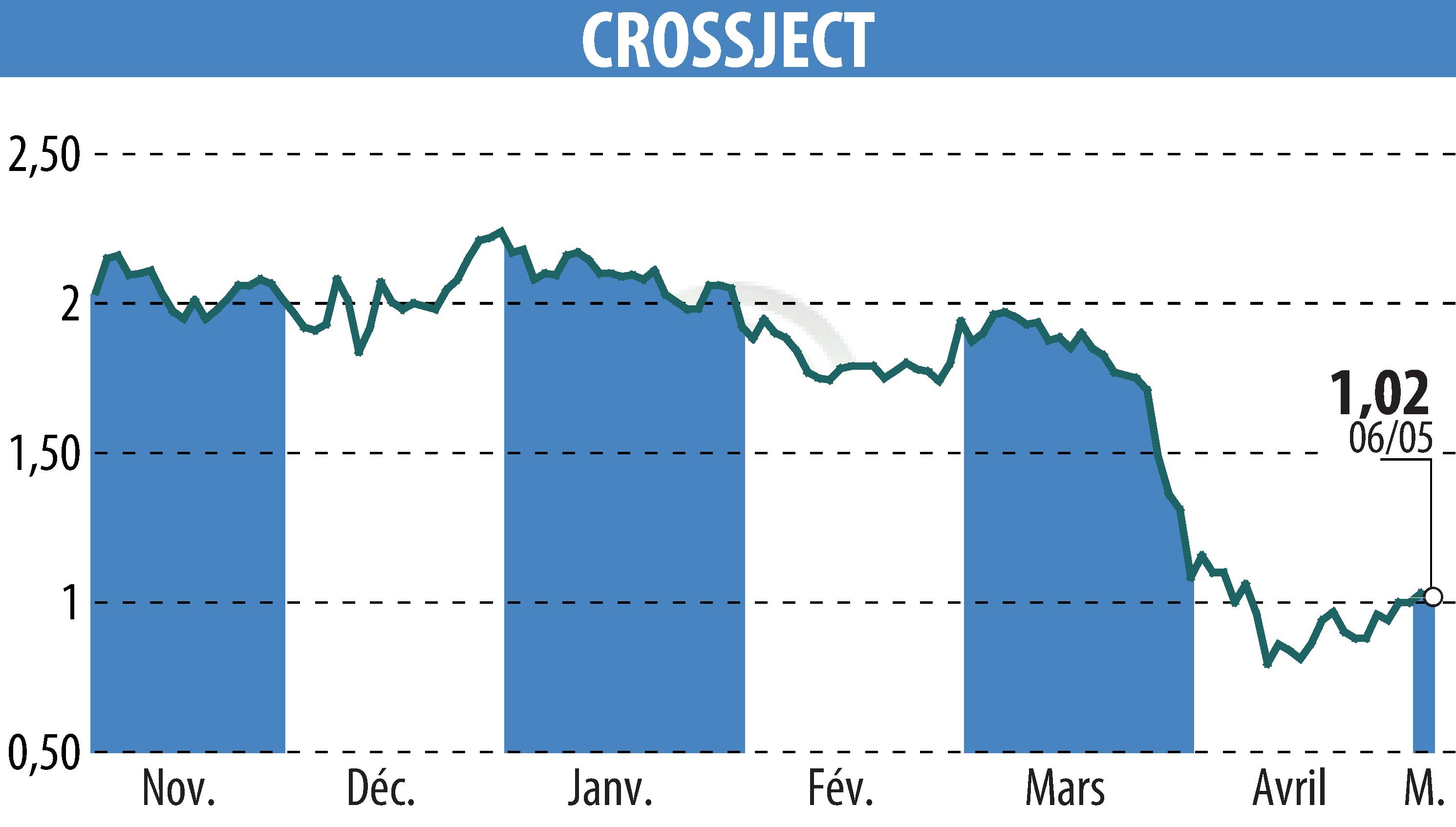
CROSSJECT announced the progress of its Emergency Use Authorization (EUA) application for its ZEPIZURE® product with the U.S. FDA. Working with EUROFINS CDMO, the company successfully completed aseptic filling of the required record batches. They plan to submit the final necessary manufacturing data by June 2025.
The company has initiated final regulatory activities to submit the ZEPIZURE® dossier under the EUA, in cooperation with BARDA, a partner in the United States. EUA batches for the CHEMPACK program for national chemical preparedness have also begun production.
CROSSJECT remains optimistic about receiving FDA clearance through its partnership with BARDA, further strengthening its ZENEO® needle-free injection technology.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all CROSSJECT news
