on CureVac (NASDAQ:CVAC)
CureVac and GSK Report Positive Phase 2 Data for Influenza mRNA Vaccine
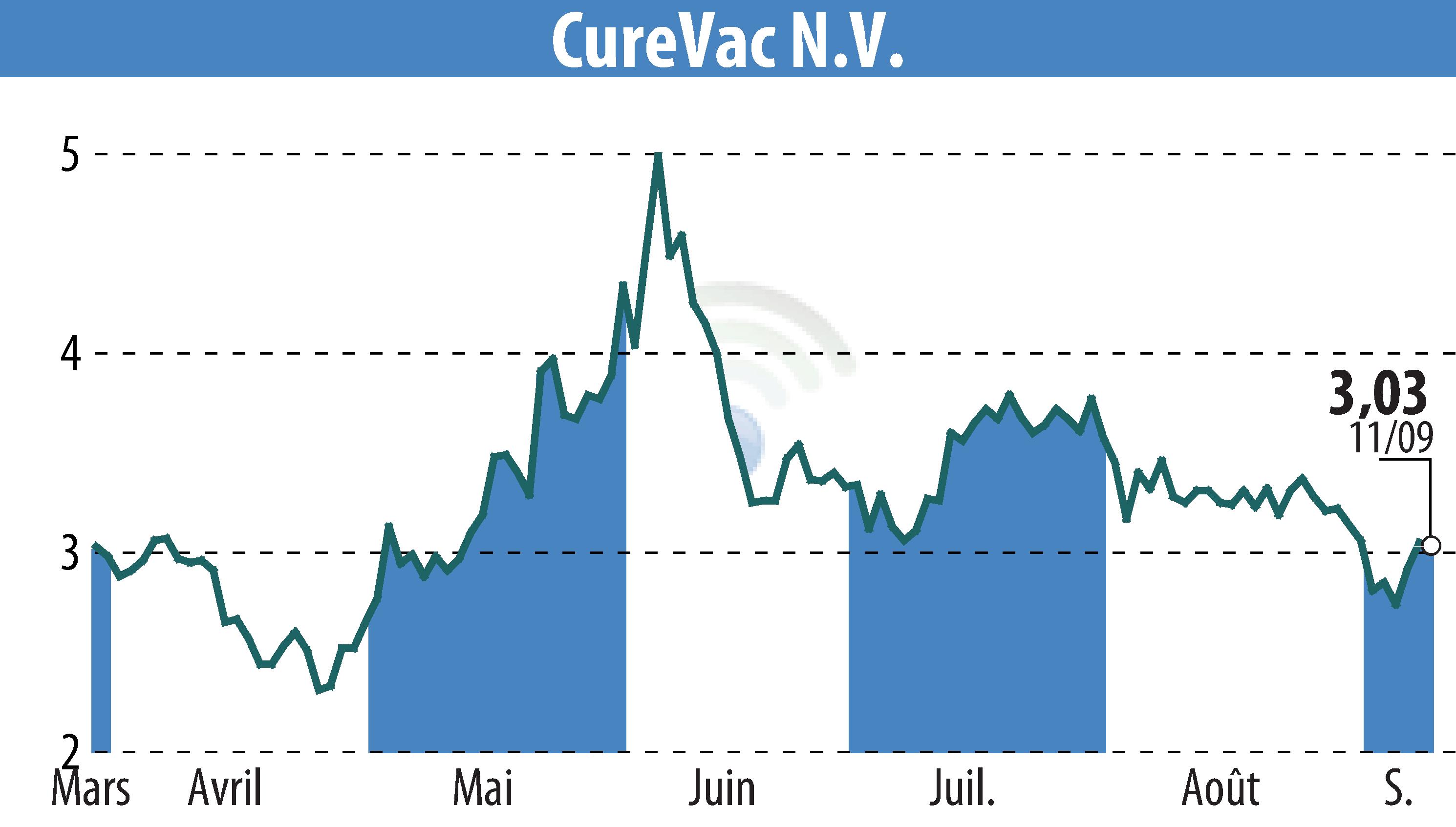
CureVac's partner GSK has announced positive Phase 2 results from their seasonal influenza mRNA vaccine program. The data showed strong immune responses to both A and B strains of influenza, meeting all predefined study endpoints. The vaccine candidate uses CureVac’s proprietary second-generation mRNA technology.
GSK's data suggests the vaccine has an acceptable safety and reactogenicity profile. This supports advancing to Phase 3, which is a significant milestone for CureVac.
In July 2024, GSK took full control of the influenza vaccine’s development, manufacturing, and commercialization under a new licensing agreement. The Phase 2 study included 500 participants, both younger and older adults, to compare immune responses.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all CureVac news
