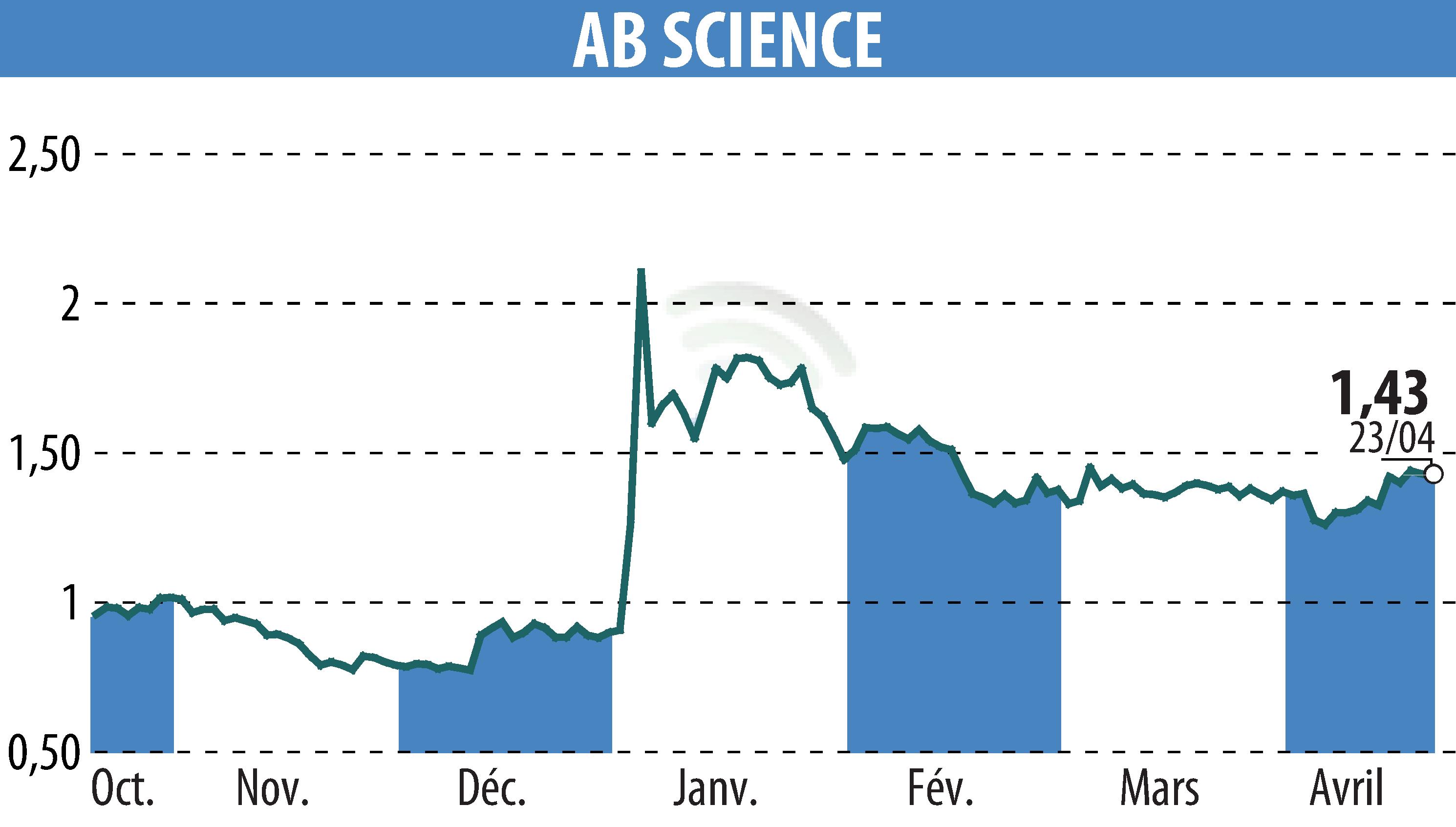
on ABSCIENCES (EPA:AB)
EMA Grants Orphan Drug Status to AB8939 for AML Treatment
The European Medicines Agency (EMA) has given orphan drug designation to AB8939, developed by AB Science, for treating acute myeloid leukemia (AML) in the EU. This designation acknowledges the drug's potential significant benefits for patients without satisfactory existing treatments.
AB8939 had previously received orphan status from the US FDA. Its effectiveness is demonstrated through preclinical mouse studies, showing advantages over current therapies like cytarabine and venetoclax, including efficacy in resistant AML cells and synergy with standard treatments without serious side effects.
The drug's designation follows stringent EMA criteria, ensuring its potential contribution to improving treatment options for AML patients, particularly those with resistant strains.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all ABSCIENCES news
