on AMOEBA (EPA:ALMIB)
Emergency authorization for Amoéba's AXPERA product in France
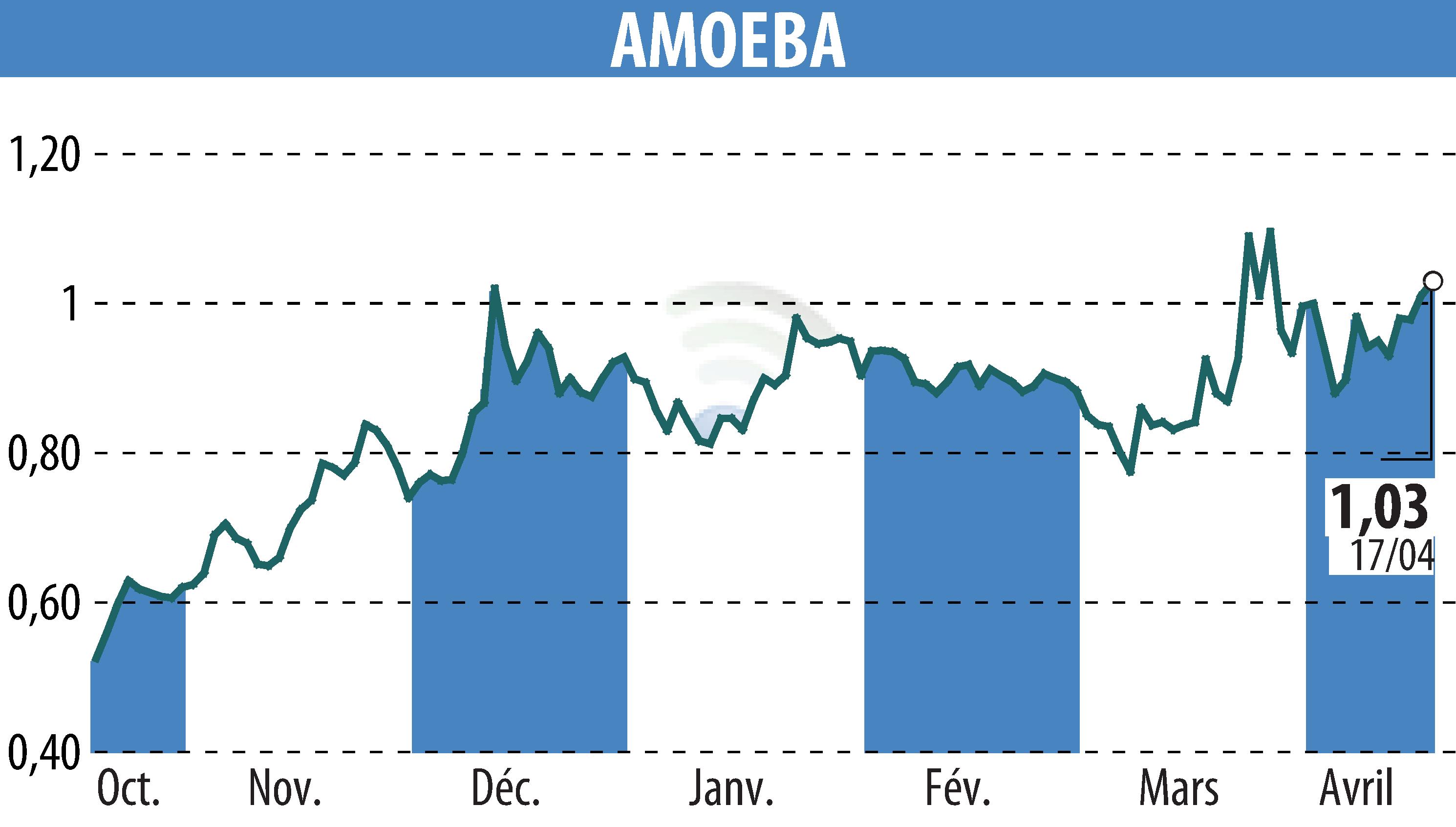
On April 22, 2025, Amoéba, a greentech company based in Chassieu, obtained emergency marketing authorization for its biocontrol product, AXPERA. This permit, granted by the Ministry of Agriculture and Food Sovereignty, will make it possible to combat grapevine mildew in 2025.
The 120-day authorization, valid from mid-April to mid-August, facilitates the integration of AXPERA into French winegrowers' programs. Grapevine downy mildew represents a phytosanitary emergency. Restrictions on copper and increased resistance to conventional treatments are of concern.
AXPERA offers an effective solution by combining low doses of copper with multiple actions, helping to counter mildew resistance. Permanent approval is planned for late 2025/early 2026.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all AMOEBA news
