on EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Completes Key Phase in enVVe Study
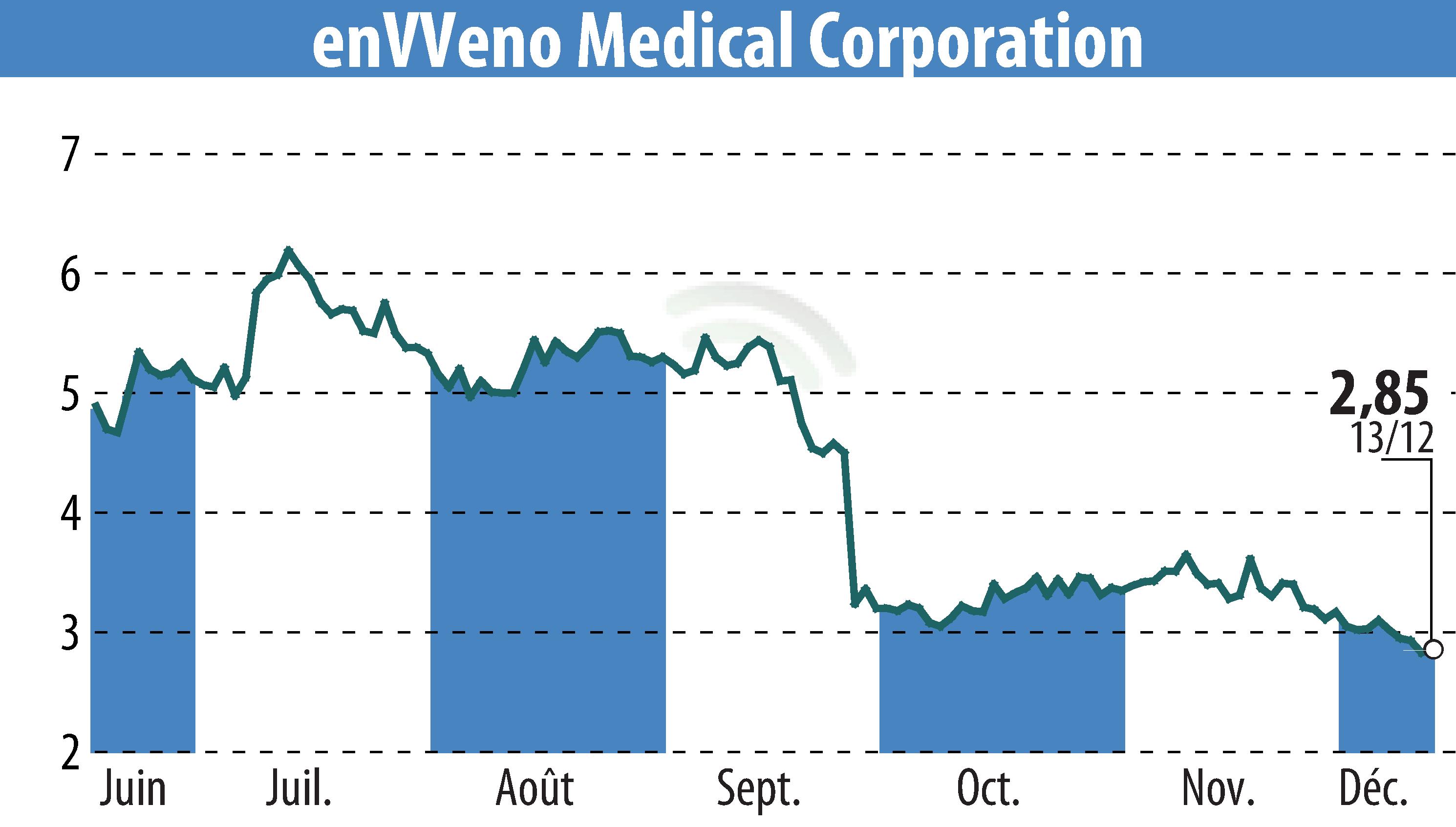
enVVeno Medical Corporation has announced the successful completion of the final wave of implants in its pre-clinical GLP study for the enVVe transcatheter-delivered venous valve. This marks a critical milestone in advancing the treatment for venous diseases. The company plans to submit an IDE application to the FDA by mid-2025, contingent on the outcomes of this study. The approval would pave the way for a pivotal trial.
CEO Robert Berman emphasized the accomplishments of the enVVe GLP study, noting the satisfactory performance of the enVVe delivery system. The aim is to establish the company as a leader in both surgical and non-surgical venous valve markets for deep venous CVI patients.
Deep venous Chronic Venous Insufficiency (CVI) is a severe condition resulting from blood clots in leg veins, leading to high treatment costs in the U.S. The company's lead product, VenoValve, is under review by the FDA, while the enVVe seeks to capture a broader market segment.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all EnVVeno Medical Corporation news
