on EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Seeks FDA Approval for VenoValve
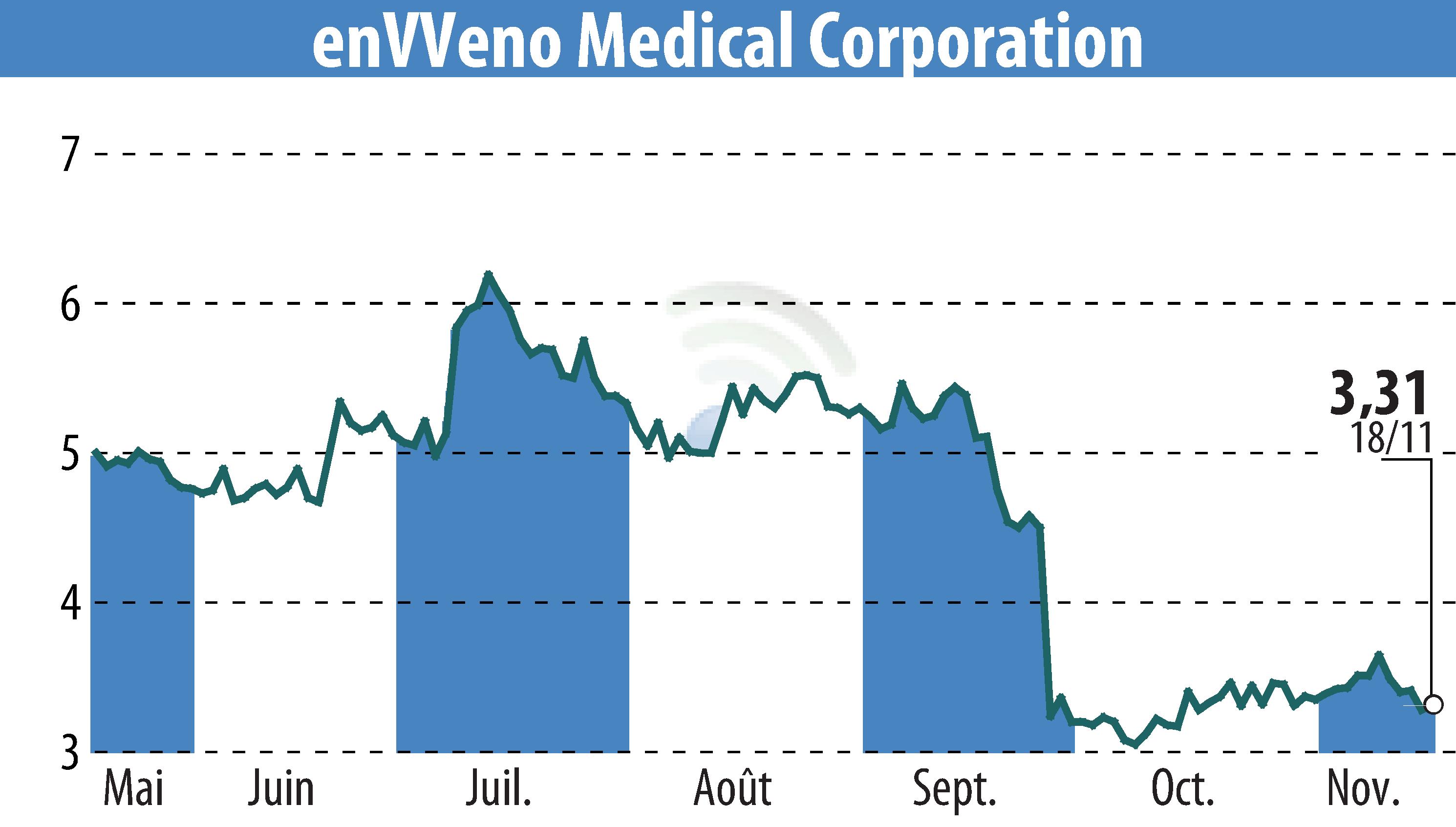
enVVeno Medical Corporation has submitted the final module of its Pre-Market Approval (PMA) application to the U.S. FDA to gain approval for the VenoValve. This application is critical for the company's plans to market and sell the device in the United States. The application comprises five modules, four of which have been approved. The final module includes clinical data from the SAVVE pivotal trial and proposed labeling information.
The VenoValve is aimed at treating severe Chronic Venous Insufficiency (CVI) caused by deep vein thrombosis. This condition affects daily life and can lead to severe symptoms such as venous ulcers. With no existing effective treatments for deep venous CVI, enVVeno estimates a potential market of 2.5 million candidates annually in the U.S.
Additionally, the company is developing a next-generation, non-surgical valve, expected to commence trials in 2025. The definitive data supporting VenoValve's PMA will be presented at the VEITH Symposium.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all EnVVeno Medical Corporation news
