on Nanohale AG (isin : DE000A1EWVY8)
European Commission Approves Formycon and Fresenius Kabi's Biosimilar FYB202/Otulfi®
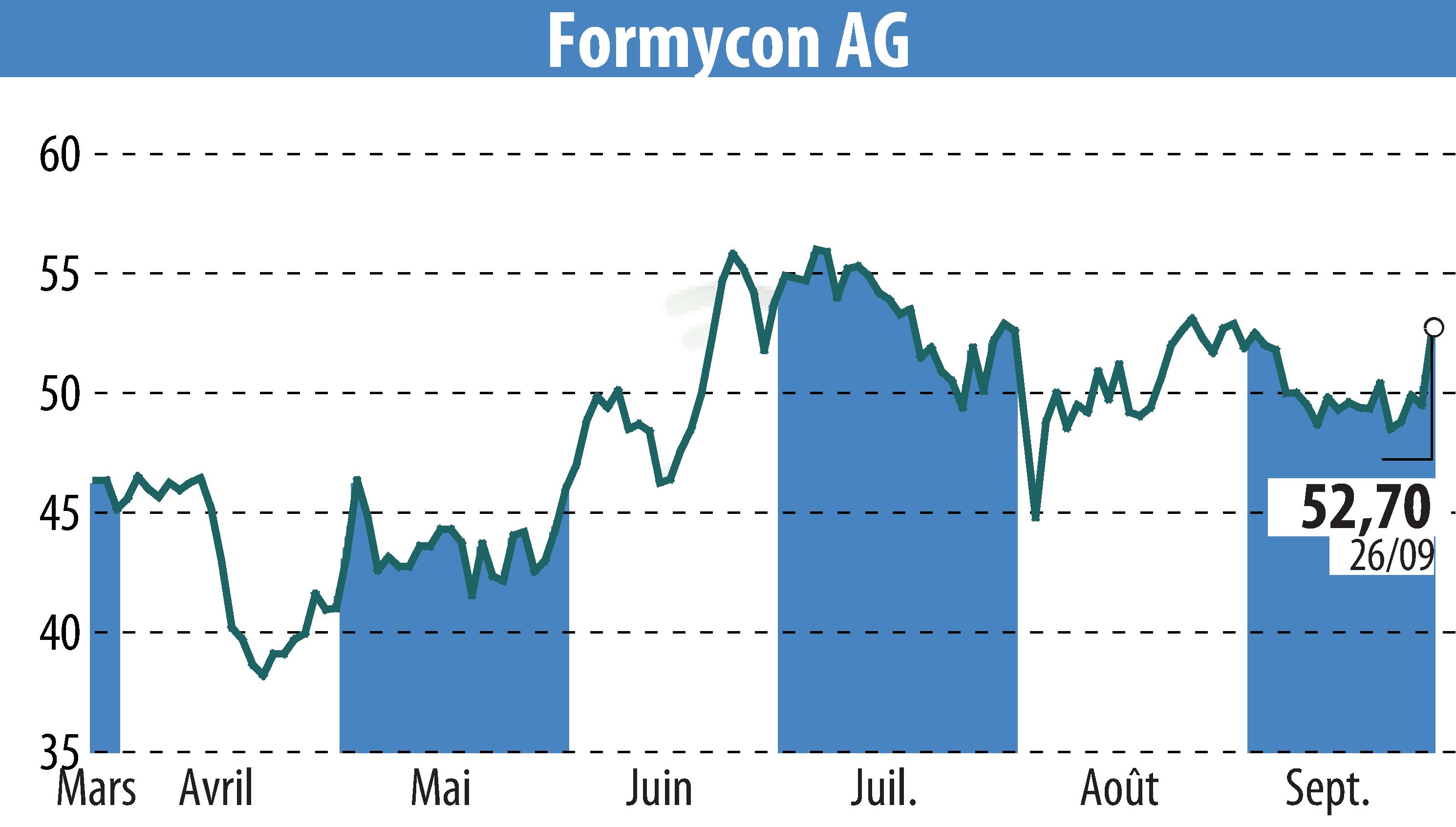
Formycon AG and its partner Fresenius Kabi have received European Commission approval for FYB202/Otulfi®, a biosimilar to Stelara®. The approval covers both subcutaneous and intravenous formulations, intended for the treatment of moderately to severely active Crohn's disease, moderate to severe plaque psoriasis, and active psoriatic arthritis.
The approval follows a positive opinion from the Committee for Medicinal Products for Human Use in July 2024. The decision is based on comprehensive data, including analytical, pre-clinical, clinical, and manufacturing evaluations. FYB202 showed comparable efficacy and safety to Stelara® in clinical trials.
Formycon and Fresenius Kabi have a global license agreement for FYB202, with semi-exclusive rights for some regions remaining with Formycon. Stelara® is one of Europe's top medicine brands, with sales exceeding €2.5 billion annually.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
