on Nanohale AG (isin : DE000A1EWVY8)
FDA Grants Approval for Stelara® Biosimilar FYB202/OtulfiTM
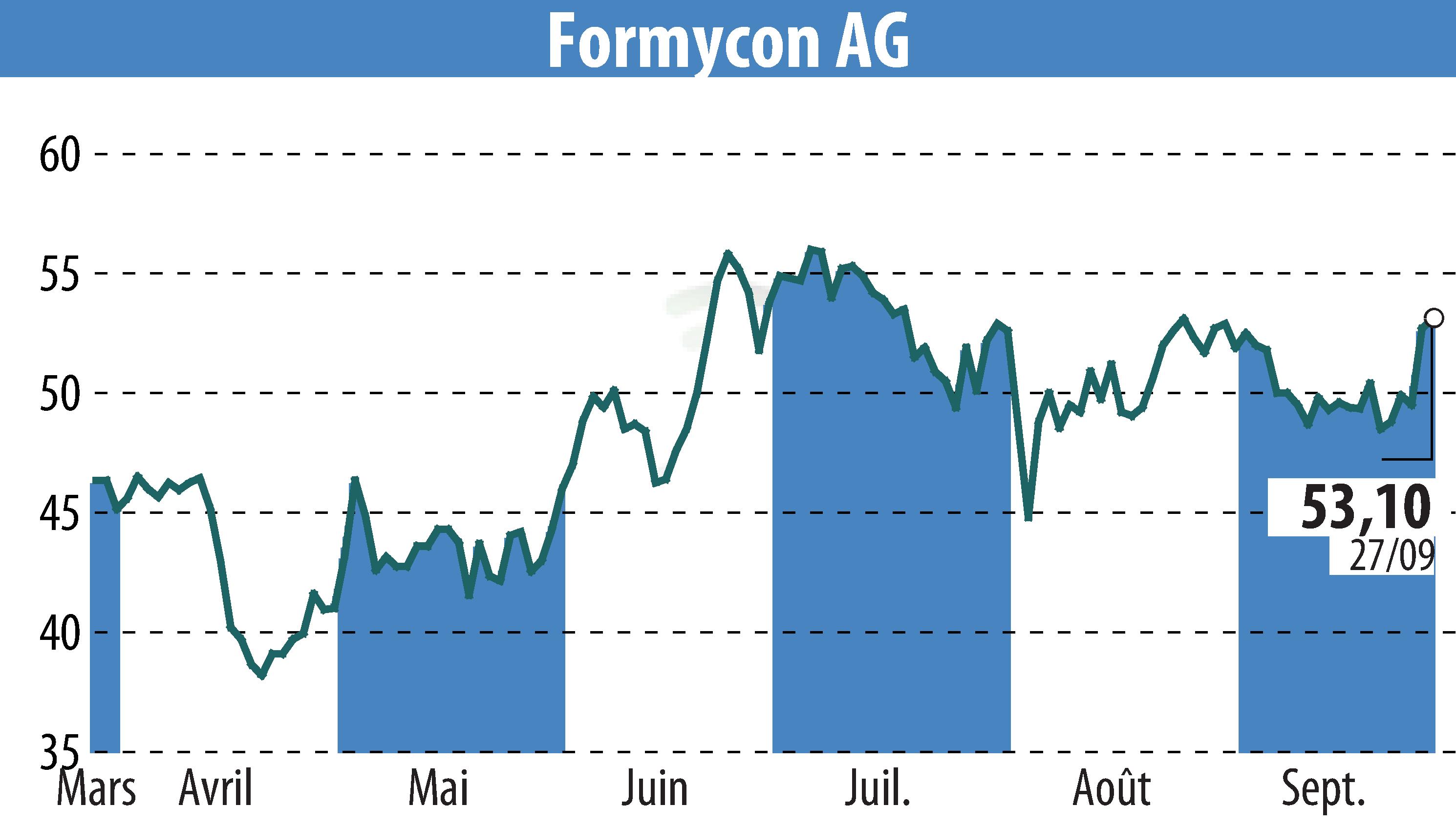
Formycon AG has announced that the U.S. Food and Drug Administration (FDA) approved FYB202/OtulfiTM, a biosimilar to Stelara®. This approval covers treatment for Crohn’s disease, ulcerative colitis, moderate-to-severe plaque psoriasis, and active psoriatic arthritis.
The approval is based on a comprehensive data package that includes analytical, pre-clinical, clinical, and manufacturing data. FYB202/OtulfiTM has shown comparable efficacy, safety, and pharmacokinetics to the reference drug Stelara® in patients with moderate to severe plaque psoriasis.
FYB202/OtulfiTM is a trademark of Fresenius Kabi Deutschland GmbH in selected countries, while Stelara® is a registered trademark of Johnson & Johnson.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
