on SANOFI-AVENTIS (EPA:SAN)
FDA Grants Breakthrough Therapy Status to Tolebrutinib for nrSPMS
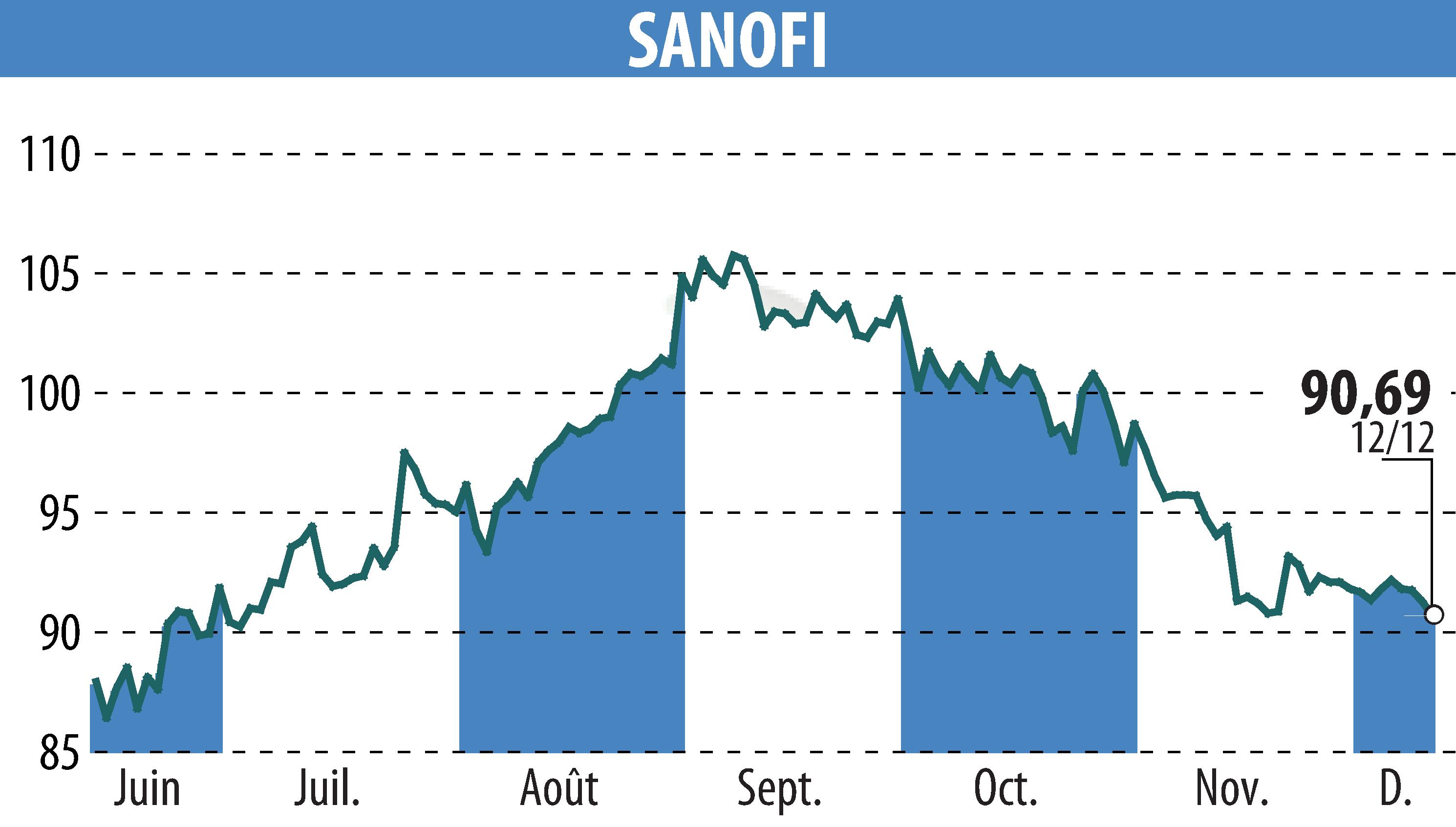
The US FDA has designated tolebrutinib as a Breakthrough Therapy for non-relapsing secondary progressive multiple sclerosis (nrSPMS). This follows positive outcomes from the HERCULES phase 3 study, showing a 31% delay in disability progression compared to placebo. Notably, tolebrutinib nearly doubled the number of participants experiencing disability improvement.
This designation aims to expedite the development of therapies for serious conditions, highlighting tolebrutinib's potential as the first brain-penetrant BTK inhibitor in this field. Liver enzyme elevations were more prevalent in the tolebrutinib group but mostly resolved without intervention.
Sanofi plans regulatory submissions for the US and EU, while the PERSEUS study continues for primary progressive MS, with results expected in late 2025.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
