on Nanohale AG (isin : DE000A1EWVY8)
Formycon and Fresenius Kabi Receive FDA Approval for Biosimilar Otulfi
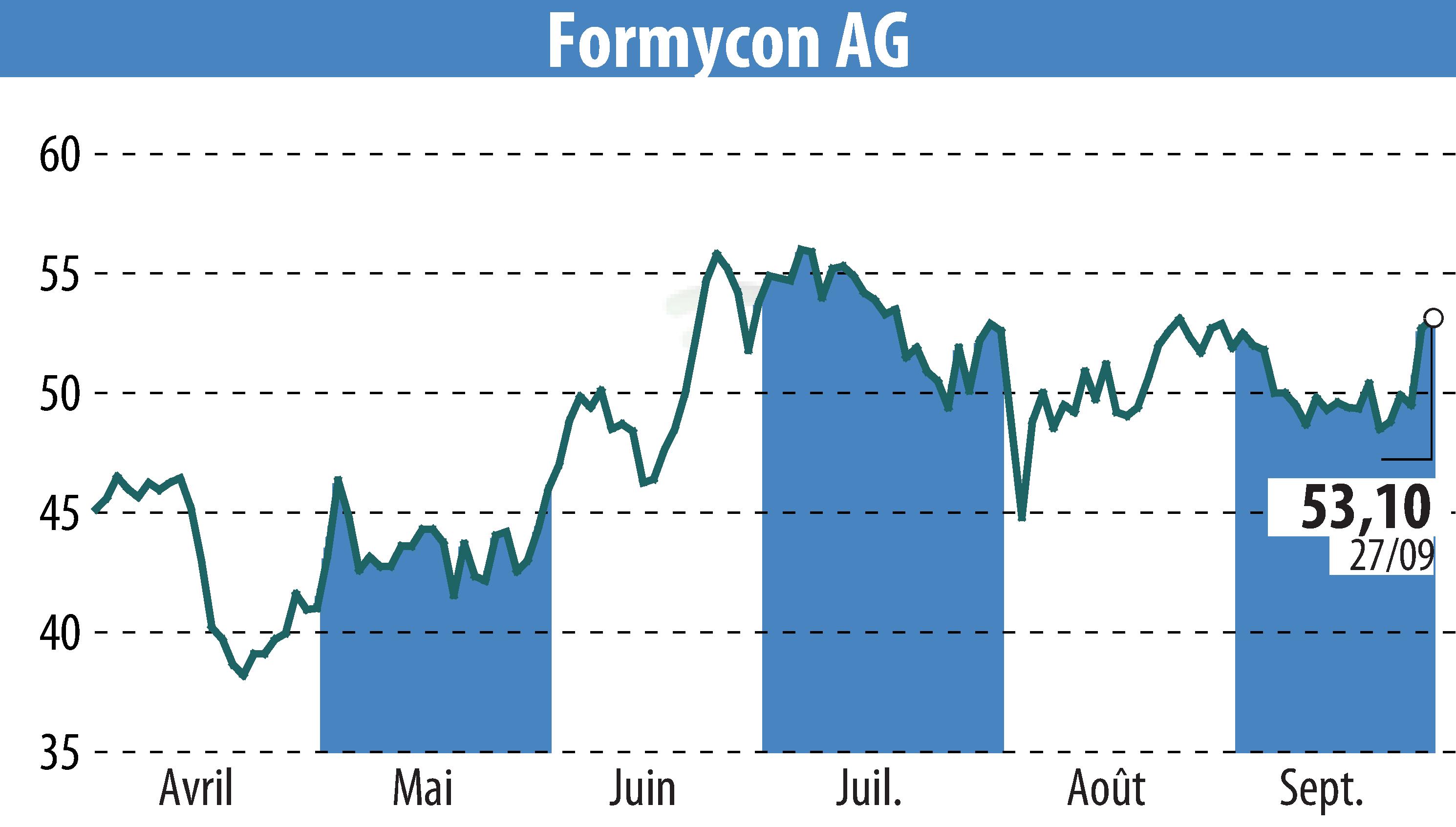
Formycon AG and Fresenius Kabi have announced the FDA approval of FYB202/Otulfi (ustekinumab-aauz), a biosimilar to Stelara, for subcutaneous and intravenous use. This marks Formycon's third FDA-approved biosimilar, with two approvals in 2024 alone. FYB202 is approved for treating Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis.
Under a patent settlement, Fresenius Kabi can market Otulfi in the US by February 22, 2025. The biosimilar has shown comparable efficacy and safety to Stelara, which earned over $10 billion in global sales in 2023.
Dr. Stefan Glombitza, CEO of Formycon, highlighted the approval as a significant milestone aligning with their goals to enhance accessibility to biologic therapies. CFO Enno Spillner emphasized the financial benefits, forecasting that FYB202 will bolster Formycon’s profitability.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
