on Nanohale AG (isin : DE000A1EWVY8)
Formycon and Fresenius Kabi Receive Positive CHMP Opinion for FYB202 (Ustekinumab)
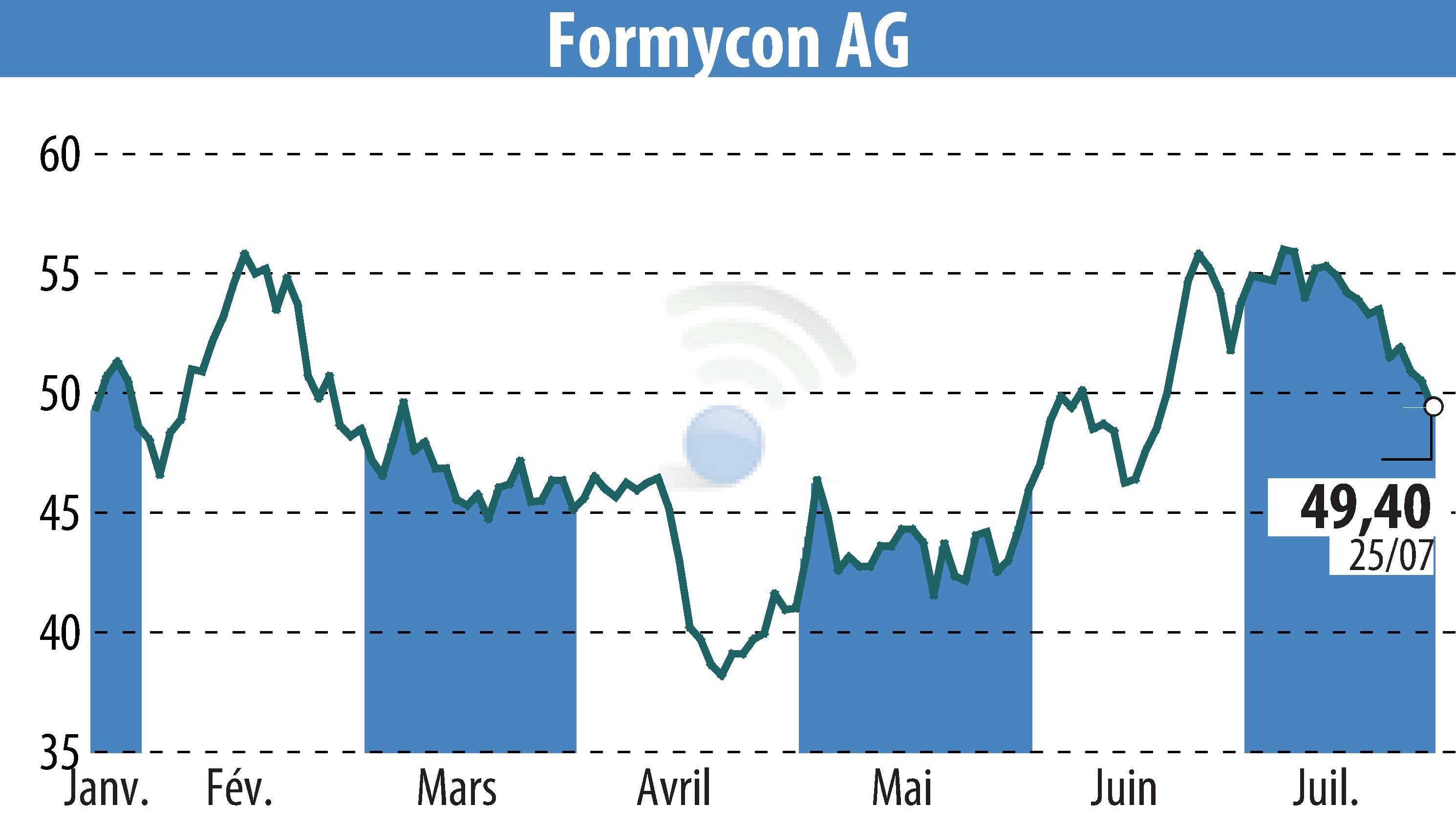
Formycon AG and Fresenius Kabi have jointly announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion for their biosimilar candidate FYB202. The candidate, Ustekinumab, is intended for the treatment of severe inflammatory diseases in fields such as gastroenterology, dermatology, and rheumatology. The European Commission is expected to make a final approval decision by early Q4 2024.
The CHMP's recommendation is a crucial regulatory step toward granting marketing authorization for FYB202 across the EU. Fresenius Kabi serves as the commercialization partner for key global markets. Dr. Stefan Glombitza, CEO of Formycon AG, emphasized that this recommendation would allow more patients access to affordable and high-quality therapies.
Ustekinumab targets interleukin-12 and interleukin-23 cytokines, tackling severe inflammatory conditions. The recommendation follows an evaluation of extensive analytical, pre-clinical, clinical, and manufacturing data proving its comparable efficacy and safety to Stelara® in patients with moderate to severe psoriasis vulgaris (plaque psoriasis).
The two companies had entered into a global commercialization partnership for Ustekinumab in February 2023, covering key global markets. The biosimilar is expected to significantly boost Formycon's revenues and contribute to sustainable profitability.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
