on Nanohale AG (isin : DE000A1EWVY8)
Formycon Unveils Clinical Data for Ustekinumab Biosimilar FYB202 at ECCO Congress
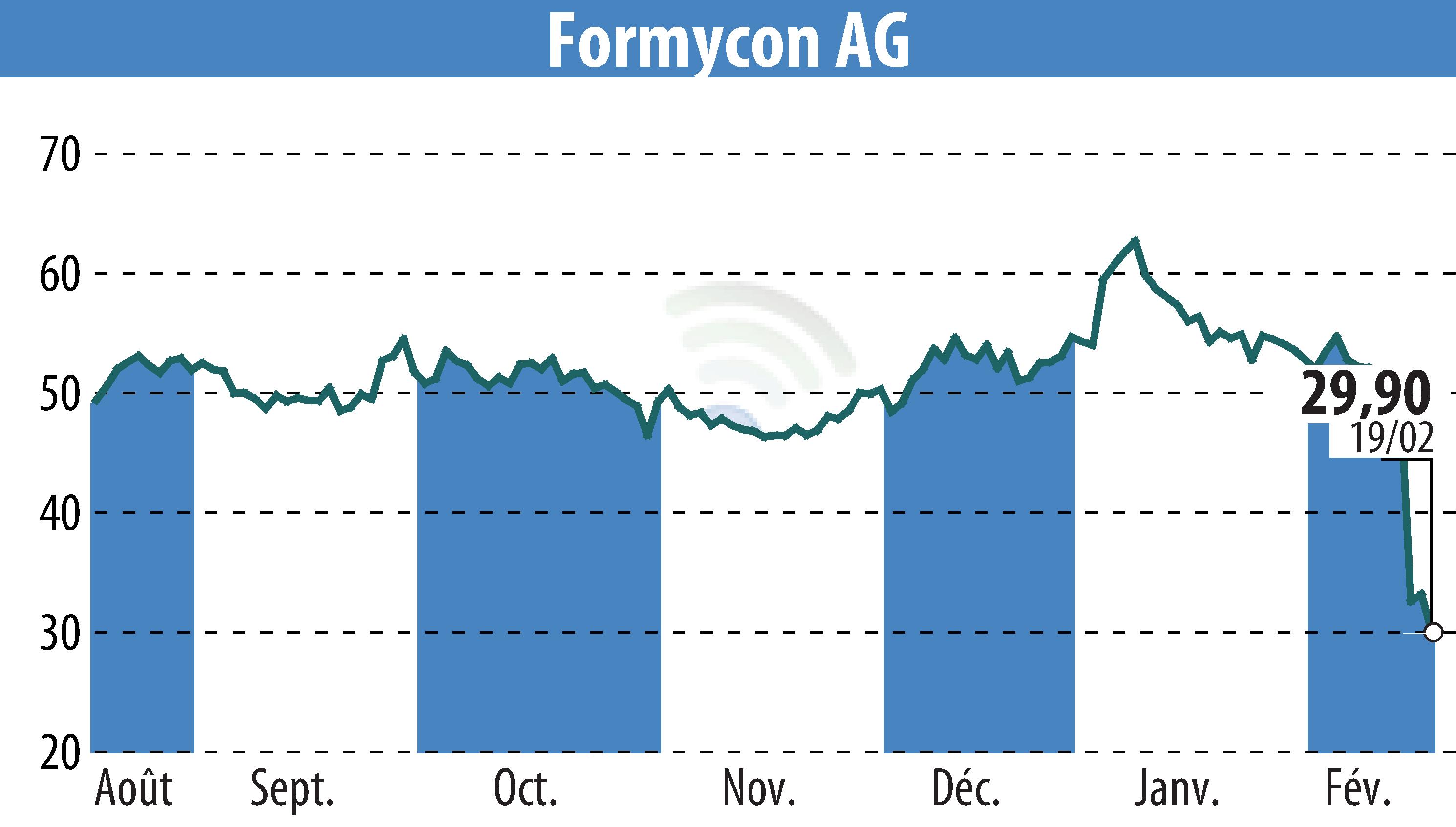
Formycon AG presented significant clinical data on its ustekinumab biosimilar, FYB202, during the European Crohn's and Colitis Organisation (ECCO) Congress in Berlin. The data presentation covered both Phase I and Phase III trial results, highlighting the bioequivalence of FYB202 to the reference drug, Stelara®.
Advanced analytical methods confirmed that FYB202 shares comparable physicochemical and biological properties with the reference, including structure and function. Therapeutic equivalence was demonstrated through pharmacokinetics studies in healthy volunteers and efficacy studies in patients with severe psoriasis. These findings provide a basis for extrapolating the biosimilar’s use to other approved indications of Stelara®.
FYB202 has received regulatory approvals from the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), Health Canada, and the UK's Medicines and Healthcare products Regulatory Agency (MHRA). This positions FYB202 as a viable alternative in treating psoriasis and chronic inflammatory bowel disease.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
