on Nanohale AG (isin : DE000A1EWVY8)
Formycon Updates on Phase III Trial and Valuation Adjustments
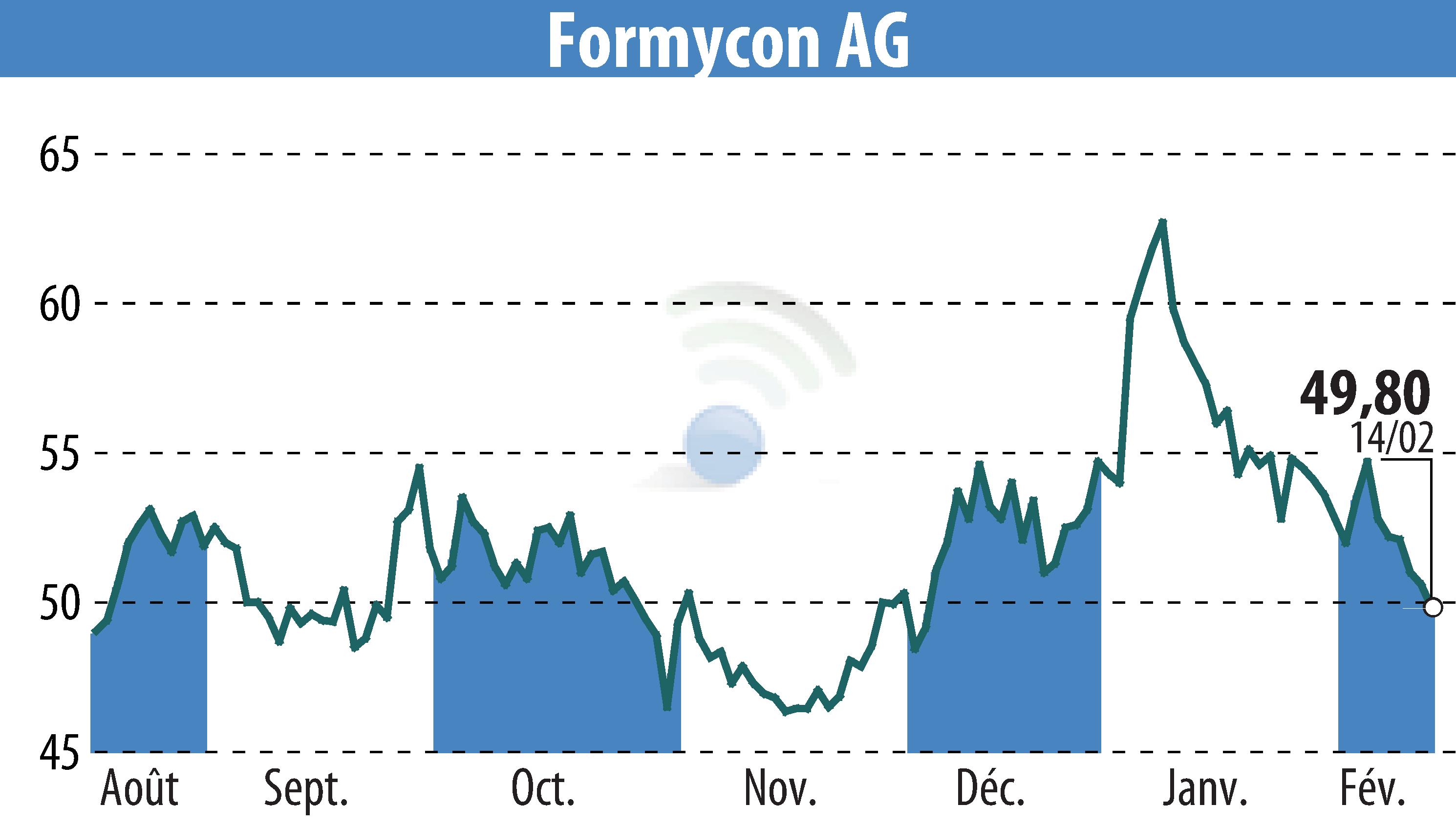
Formycon AG has concluded its Phase III "Lotus" trial for the biosimilar FYB206 earlier than expected, following positive feedback from the FDA. Data from an ongoing melanoma study, combined with analytical results, sufficiently demonstrates therapeutic comparability to Keytruda®. This decision is projected to save significant investments, benefiting Formycon's future cash flow.
With the U.S. launch of FYB202/Otulfi imminent, Formycon anticipates the need to adjust its valuation models due to unexpected large price discounts for biosimilars. Preliminary calculations suggest a potential non-cash impairment within a high double-digit to low triple-digit million range.
Regarding FYB201/CIMERLI®, due to pricing pressures, discussions with Sandoz AG may lead to a temporary commercialization pause in the U.S. This would necessitate further adjustments to Formycon's financial models for FYB201.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
