on Nanohale AG (isin : DE000A1EWVY8)
Formycon's Breakthrough in Biosimilar Development
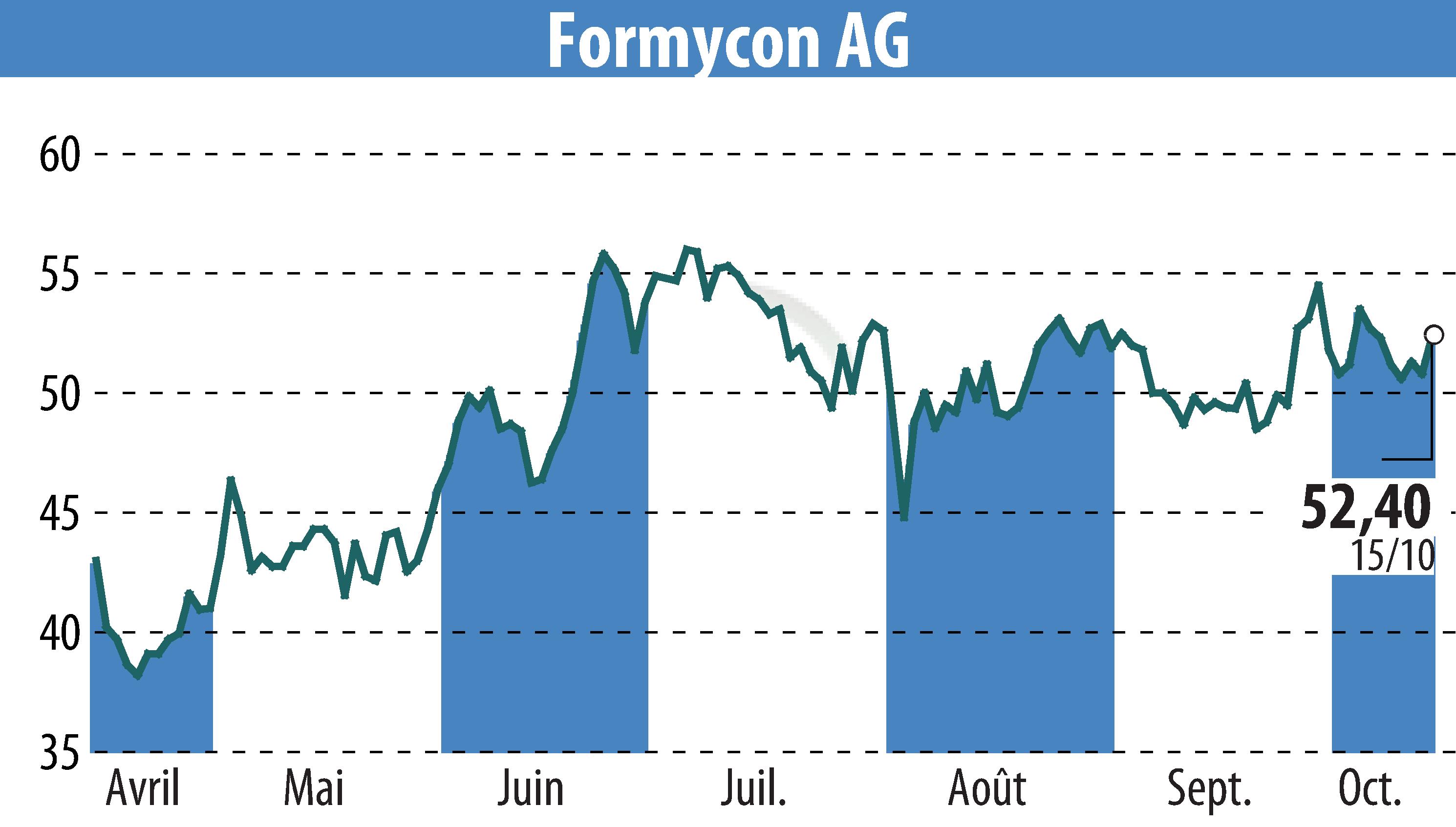
Formycon AG has published promising results from its analytical similarity study of the biosimilar candidate FYB206 compared to Keytruda®, as detailed in the peer-reviewed journal Drugs in R&D. The study found that FYB206 is structurally and functionally highly similar to Keytruda®, indicating a strong potential for clinical success.
With these results, FYB206's ongoing clinical trials are bolstered, supporting Formycon's position among leading developers of pembrolizumab biosimilars. A broad array of analytical tests confirmed FYB206’s similarity to Keytruda® in key functional attributes, such as PD-1 binding and neutralization.
The trials, "Dahlia" and "Lotus," are currently assessing the pharmacokinetics and efficacy of FYB206 in cancer treatments, with successful outcomes anticipated to enhance patient access to cost-effective immuno-oncology therapies.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
