on Nanohale AG (isin : DE000A1EWVY8)
Formycon's FYB203 Receives UK Regulatory Approval
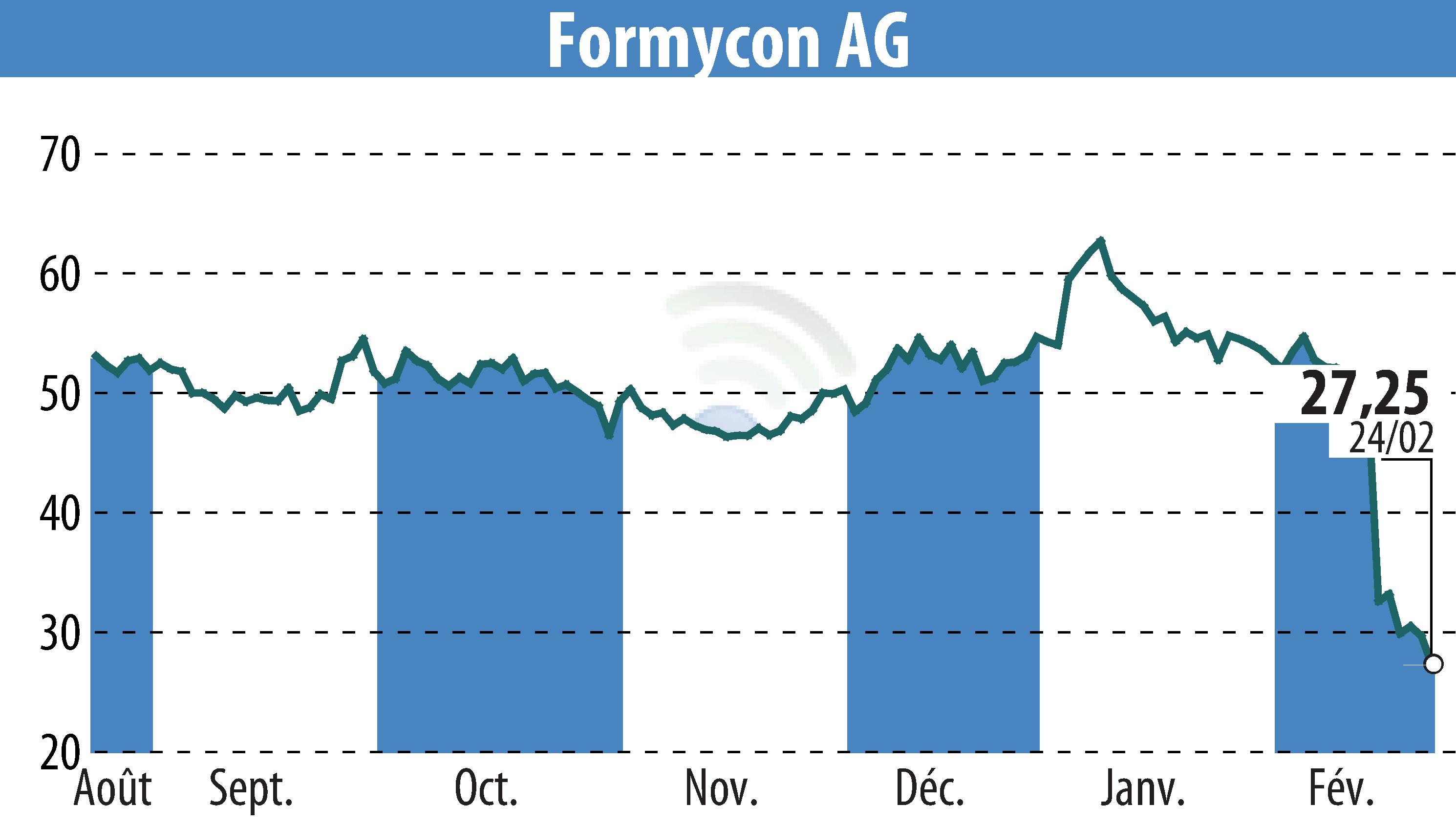
Formycon AG has announced that its biosimilar FYB203 (aflibercept), marketed under the brand name AHZANTIVE®, has received regulatory approval in the UK. This approval by the Medicines and Healthcare products Regulatory Agency (MHRA) allows FYB203 to be used for treating neovascular age-related macular degeneration (nAMD) and other severe retinal diseases.
This follows successful approvals from the U.S. FDA and the European Commission. Teva Pharmaceuticals will manage the commercialization in Europe, including the UK. This partnership capitalizes on Teva's established presence in ophthalmic treatments.
FYB203 functions as a vascular endothelial growth factor (VEGF) inhibitor, targeting the abnormal blood vessel formation in the retina, which leads to vision issues. The approval enhances options for patients needing cost-efficient treatments for retinal conditions.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
