on Nanohale AG (isin : DE000A1EWVY8)
FYB202 Biosimilar to Stelara Receives Positive CHMP Opinion from EMA
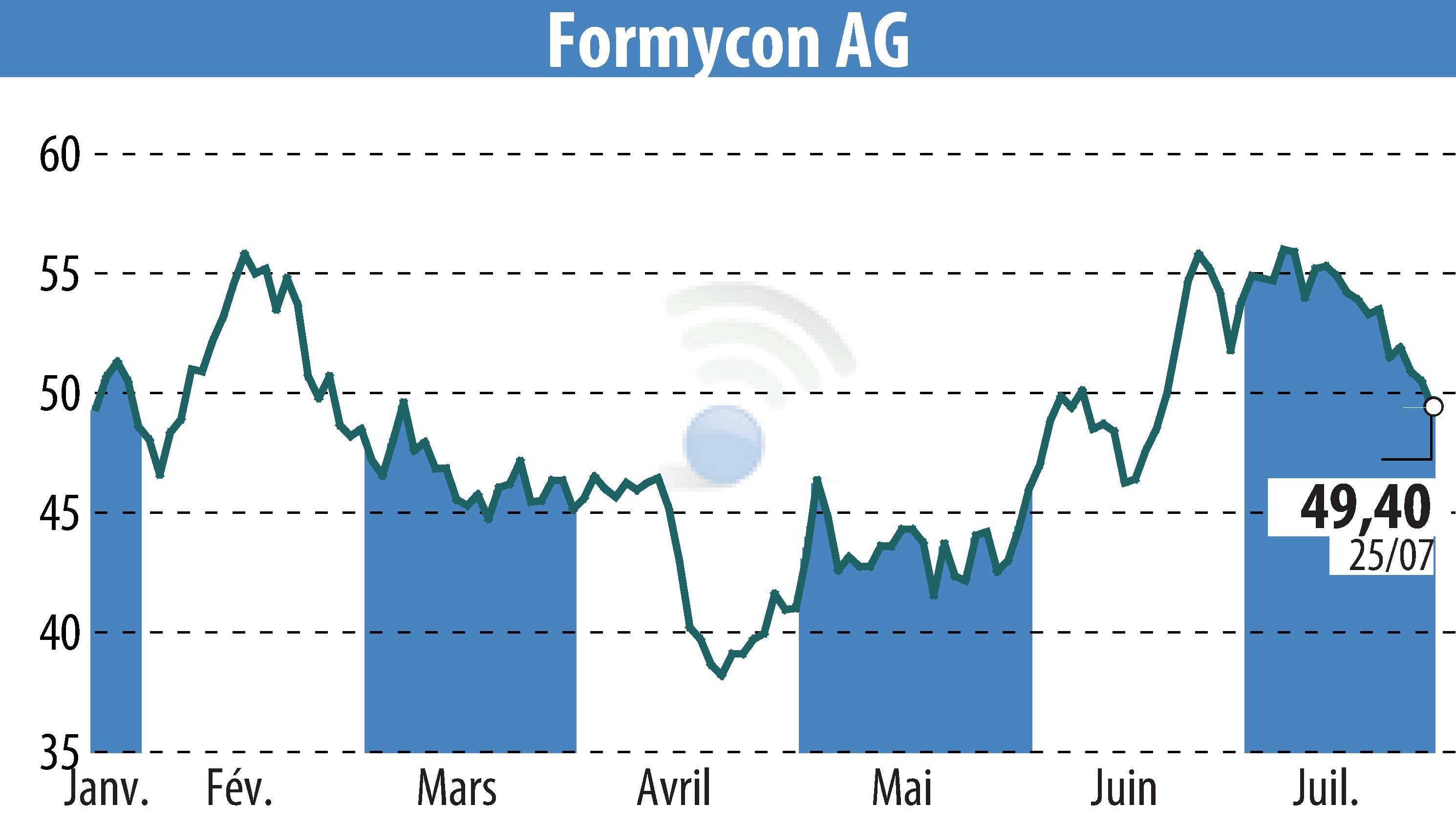
Formycon AG has announced that the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for FYB202. FYB202 is a biosimilar candidate to Stelara® (Ustekinumab), aimed at treating severe inflammatory diseases in gastroenterology, dermatology, and rheumatology.
The CHMP's favorable evaluation included a thorough review of analytical, pre-clinical, clinical, and manufacturing data. FYB202 demonstrated comparable efficacy, safety, and pharmacokinetics to Stelara® in patients with moderate to severe plaque psoriasis. This recommendation paves the way for the European Commission's decision, expected by early Q4 2024.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
