on Nanohale AG (isin : DE000A1EWVY8)
FYB202/Otulfi® Biosimilar Launched in the U.S. and EU
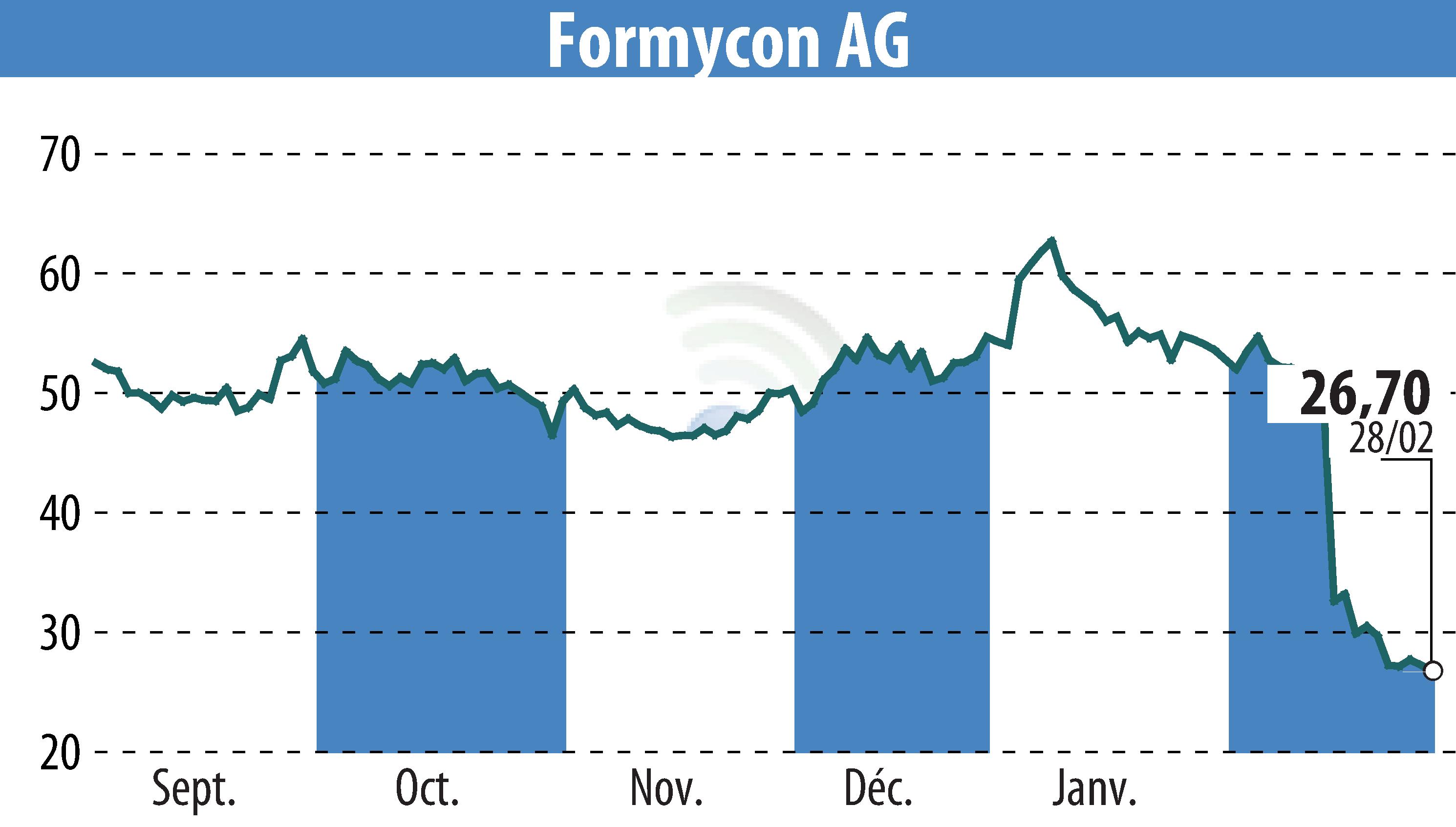
Formycon AG and Fresenius have announced the launch of FYB202/Otulfi® (ustekinumab-aauz), a biosimilar to Stelara®, now commercially available in the United States and the European Union. Otulfi® is provided in subcutaneous and intravenous forms. It aims to facilitate the transition of patients from the original biologic with equivalent efficacy and safety. Formycon and Fresenius's dedicated sales team will work to ensure access and reimbursement in key markets.
In the United States, Otulfi® treats Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis. The FDA indicates that it may soon receive an interchangeable designation with Stelara®. In the EU, it addresses Crohn’s disease, plaque psoriasis, and psoriatic arthritis.
This launch follows a 2023 global license agreement with Fresenius Kabi, granting commercialization rights in significant markets. Otulfi®’s approval by the FDA and EMA in September 2024 confirms its biosimilarity to Stelara®, offering a lower-cost treatment option for patients with chronic inflammatory diseases.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
