on GENFIT (EPA:GNFT)
GENFIT: Positive Opinion from EMA Committee for Ipsen’s Iqirvo® in Primary Biliary Cholangitis
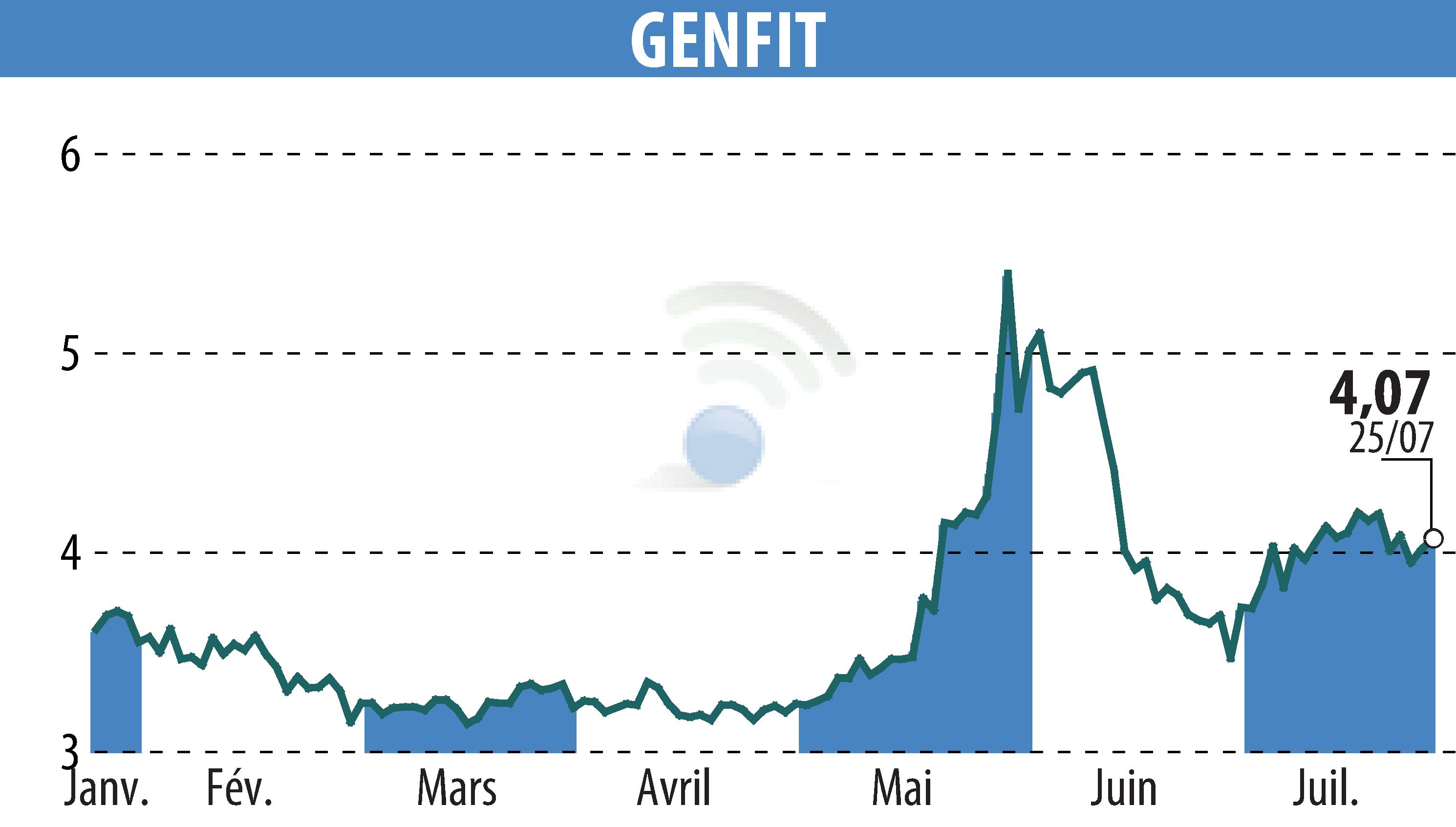
GENFIT, a late-stage biopharmaceutical company, has announced a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) regarding Ipsen’s Iqirvo® (elafibranor) for the treatment of Primary Biliary Cholangitis (PBC). This concerns its use with ursodeoxycholic acid (UDCA) in adults with inadequate UDCA response or as a monotherapy for those unable to tolerate UDCA.
Elafibranor, a 'first-in-class' molecule, is marketed and commercialized in the U.S. by Ipsen under the name Iqirvo® since June 2024. Developed by GENFIT, the drug completed a 52-week Phase 3 clinical study. Ipsen secured the exclusive worldwide rights (excluding China, Hong Kong, Taiwan, and Macau) to elafibranor from GENFIT in 2021.
The European Commission will now deliberate on the CHMP's recommendations, with a final decision on the marketing authorization for Iqirvo® expected in the second half of 2024.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GENFIT news
