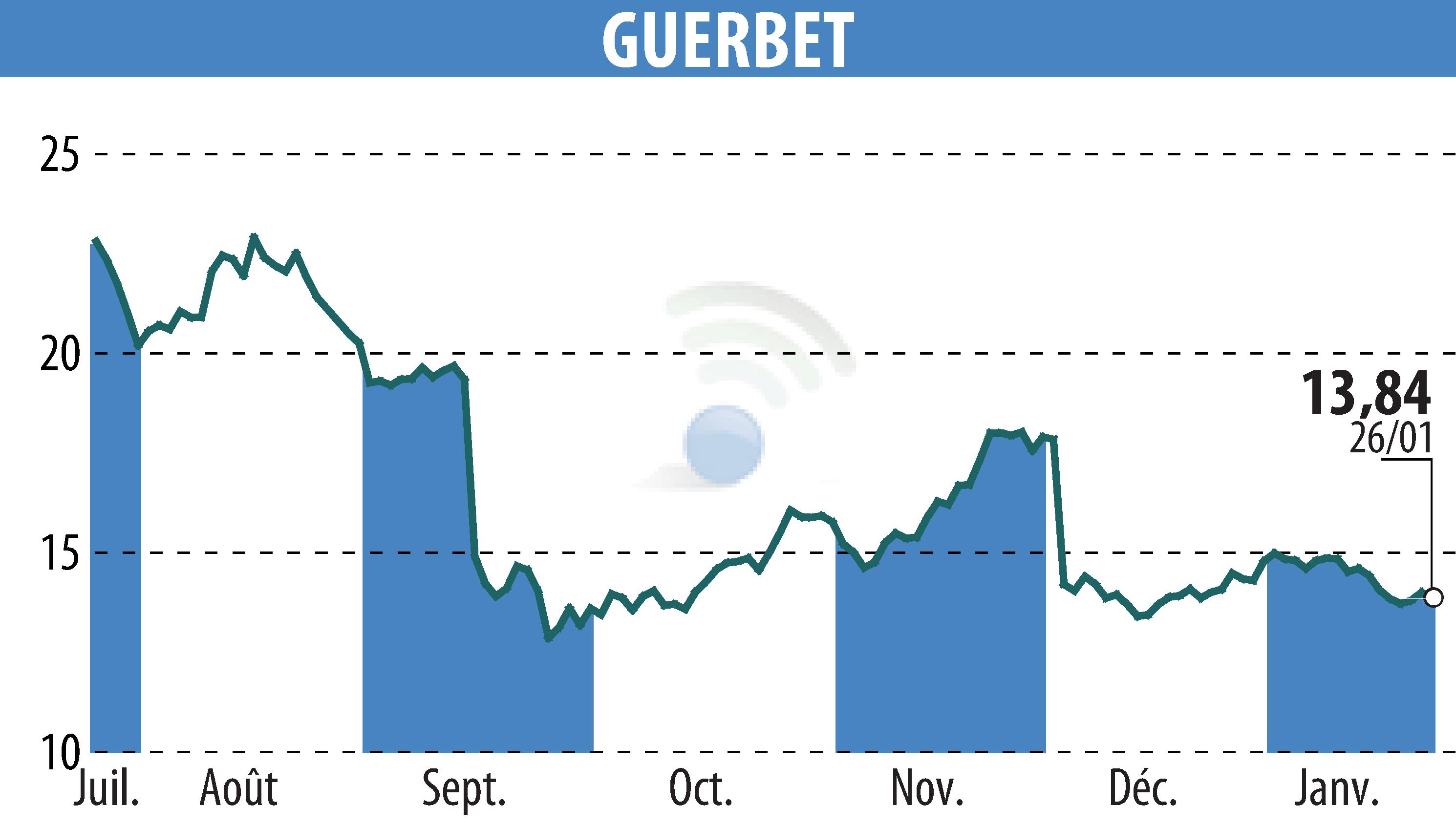
on GUERBET (EPA:GBT)
European Approval for Guerbet's Elucirem™ Use in Children from Birth
Guerbet, a leader in medical imaging solutions, has received European Commission approval for a new indication of its gadolinium-based contrast agent, Elucirem™ (Gadopiclenol), now usable in children from birth. This approval marks a significant advancement as Elucirem™ is noted for its high relaxivity, offering improved image quality at half the conventional gadolinium dose.
First approved in the EU in December 2023, Elucirem™ is already used in adults for contrast-enhanced MRIs of the central nervous system and major organs. The lower dose requirement is particularly valuable for pediatric patients, reducing cumulative gadolinium exposure.
Valérie Brissart, Guerbet's SVP, underlined the commitment to medical innovation and patient safety, emphasizing the reduction in gadolinium exposure during serial MRI examinations. Dr. Emilio J Inarejos Clemente from Hospital Sant Joan de Déu noted the benefit of high-quality imaging with minimized gadolinium exposure for vulnerable pediatric patients.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all GUERBET news
