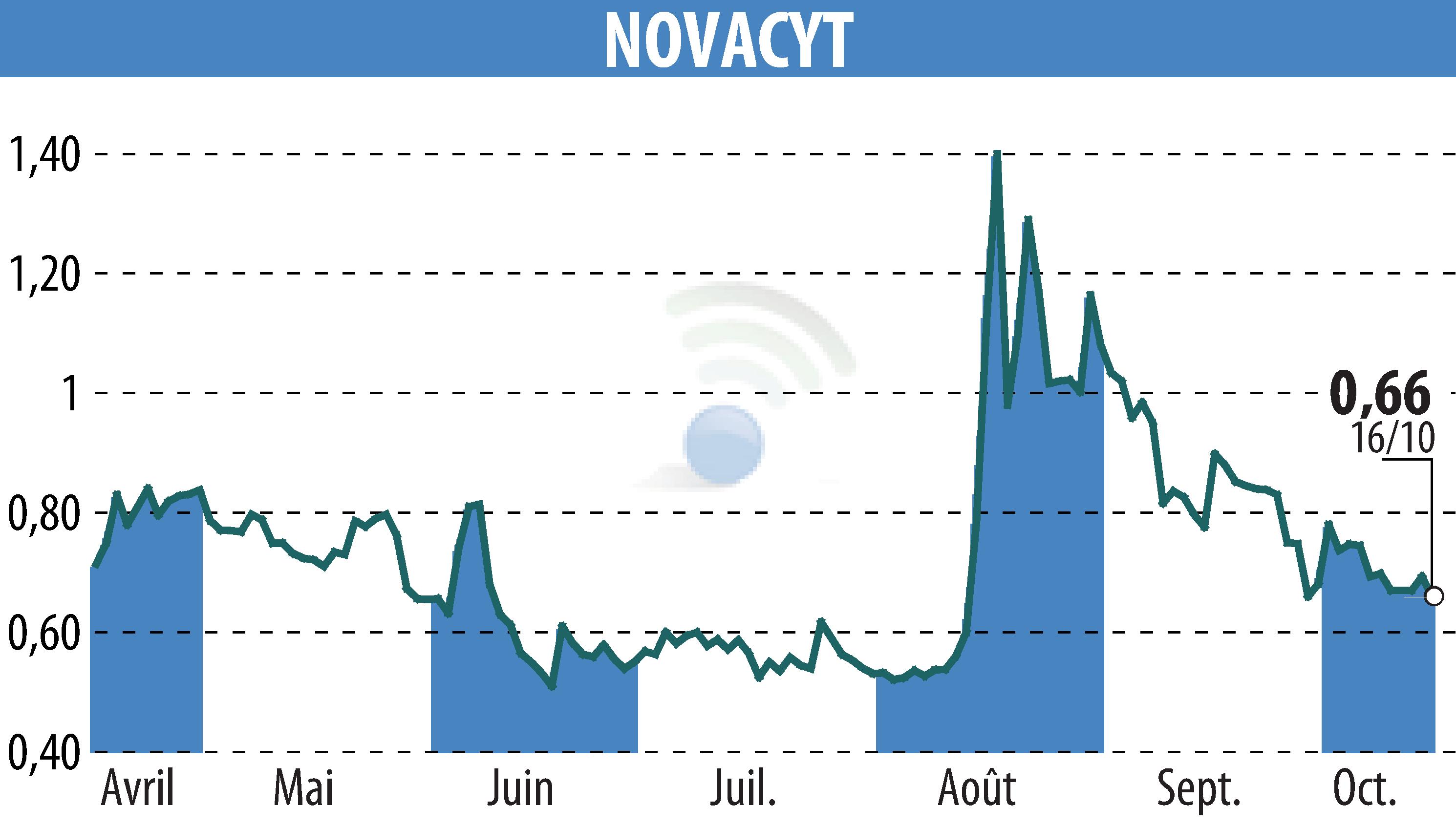
on NOVACYT (EPA:ALNOV)
IVDR Certification for Yourgene® Cystic Fibrosis Test by Novacyt
Novacyt SA has announced that it has obtained IVDR certification for its Yourgene® cystic fibrosis test, intended for healthcare professionals in molecular laboratories. This Class C in vitro test has been assessed by the British Standards Institution and found to comply with the strict requirements of European regulations. The certification ensures the quality, safety and performance of the test, manufactured for the European market.
Cystic fibrosis is a common genetic disease affecting 1 in 2,500 Caucasian newborns. In the UK, screening is carried out within the first three days of a baby’s life. The Yourgene® test uses ARMS technology to detect mutations in DNA and is designed for a variety of clinical scenarios.
This test, part of Yourgene’s reproductive health portfolio, identifies 50 common cystic fibrosis mutations. It is the second Novacyt product to achieve IVDR certification. Compliance strengthens clinicians’ and patients’ confidence in the quality of the test.
R. E.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NOVACYT news
