on Jaguar Health (NASDAQ:JAGX)
Jaguar Health Reports Phase 3 OnTarget Trial Results for Crofelemer
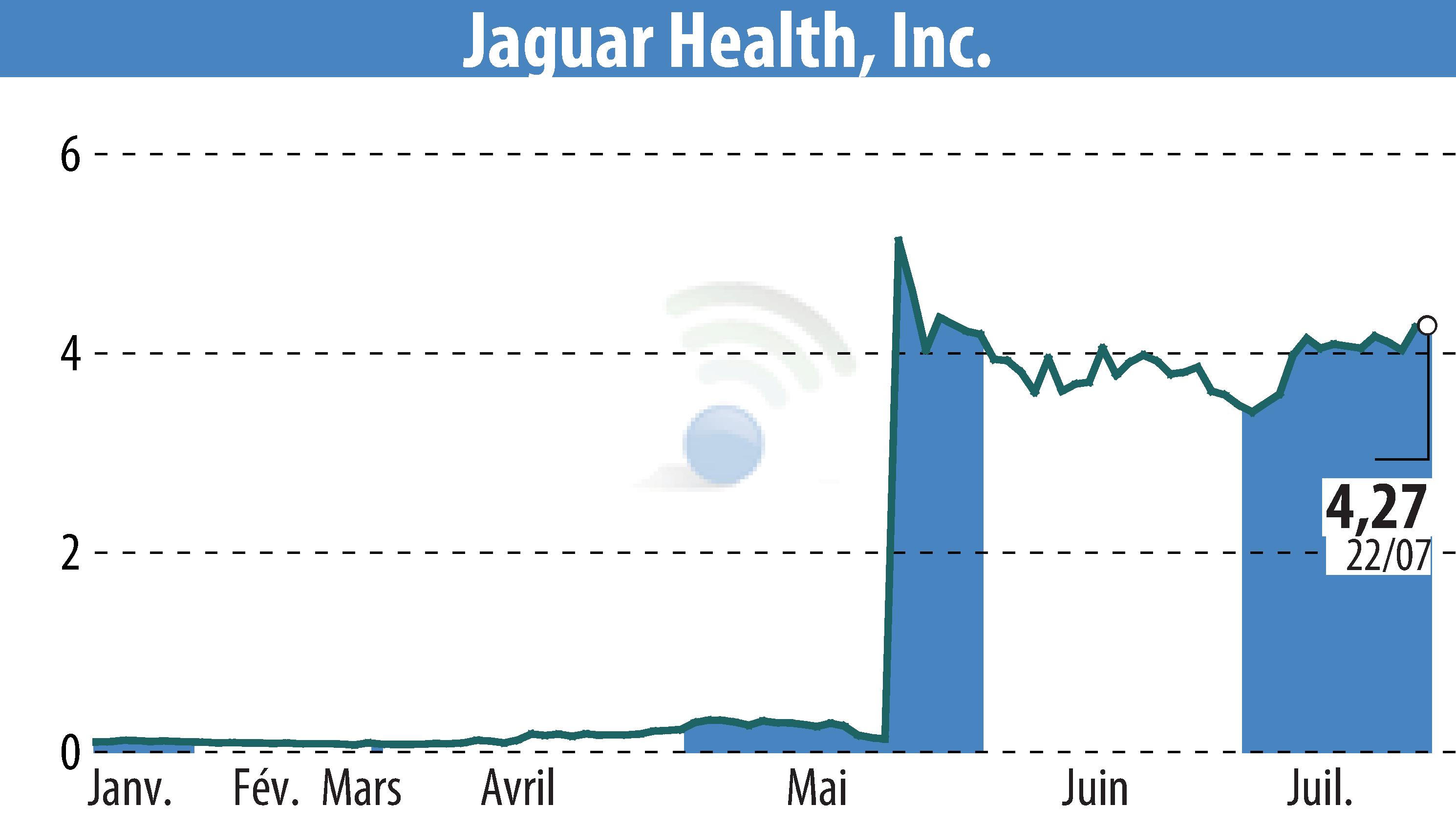
Jaguar Health announced initial results from its Phase 3 OnTarget trial of crofelemer for cancer supportive care. The study aimed to prevent diarrhea in adult cancer patients receiving targeted therapy. The trial included 10 different tumor types and failed to meet its primary endpoint across all tumor types.
However, the trial revealed clinically relevant signals for breast and respiratory cancer subgroups. These subgroups accounted for over 75% of the trial participants. The company plans to analyze data from the 12-week extension phase and engage with the FDA for future steps.
Crofelemer is already FDA-approved for treating diarrhea in adult HIV/AIDS patients. Jaguar Health aims to expand its use to cancer patients. The OnTarget trial highlights the need for novel treatments for cancer therapy-related diarrhea (CTD).
An investor webcast is scheduled for July 23rd to discuss these findings. The webcast will feature Jaguar's scientific team and oncology experts.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Jaguar Health news
