on MEDINCELL (EPA:MEDCL)
Medincell: Promising advances for injectable Olanzapine
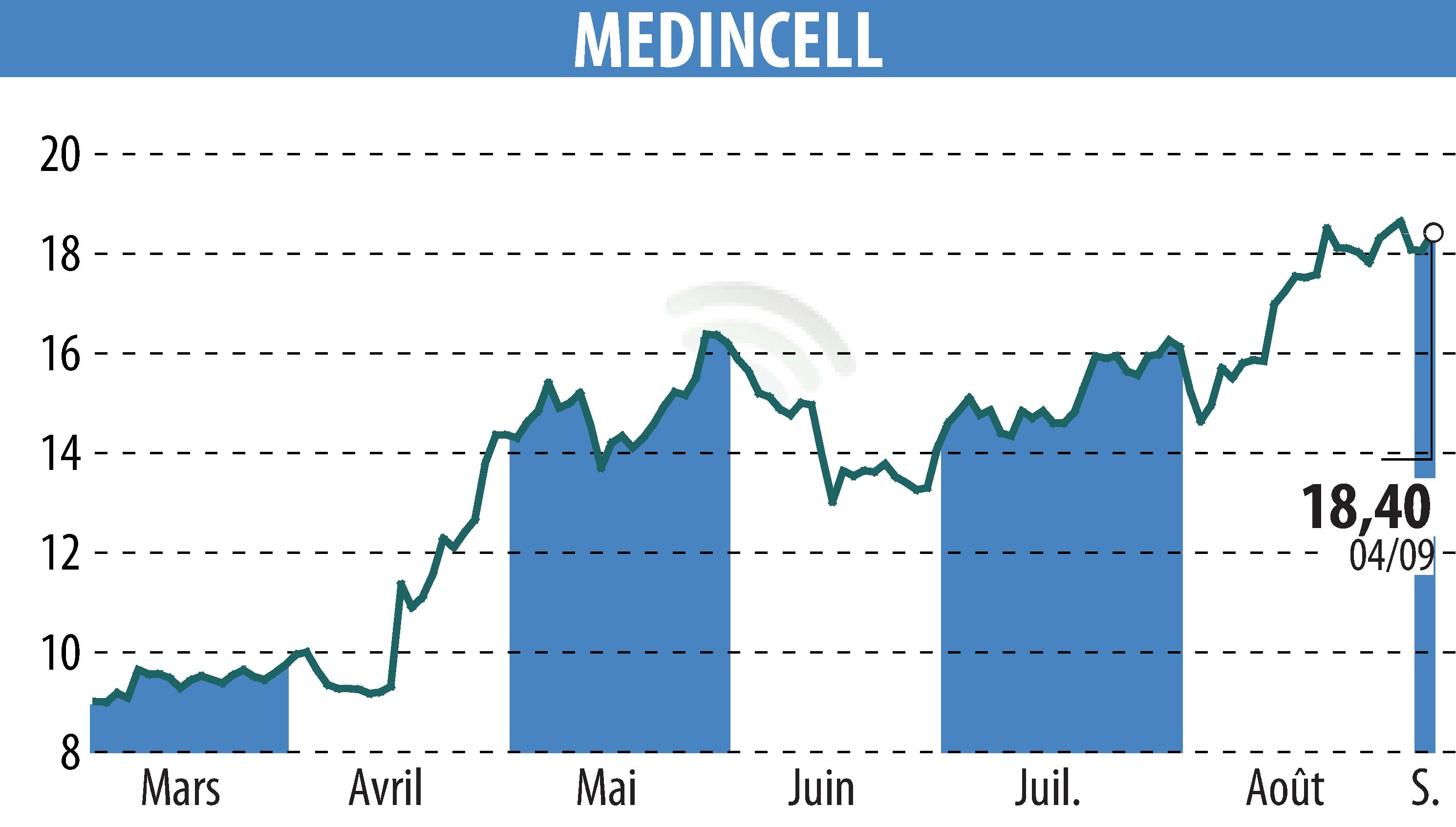
Teva Pharmaceuticals, in collaboration with Medincell, announced significant progress in the Phase 3 clinical trial for Olanzapine Long-Acting Injection (LAI). Approximately 99% of injections planned for approval were completed without the observation of post-injection sedation syndrome (PDSS).
Full safety data, essential for approval, are expected in the second half of 2024. According to results published in May 2024, the efficacy of Olanzapine LAI has been confirmed.
This treatment, administered monthly, could become the first without an FDA "black box" warning for PDSS, unlike other existing Olanzapine formulations. Teva is responsible for its development and marketing.
Medincell could receive up to $117 million in development and commercialization milestones for this program, in addition to royalties on net sales.
R. P.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDINCELL news
