on MIRA Pharmaceuticals (NASDAQ:MIRA)
MIRA Pharmaceuticals Advances Towards Clinical Trials with Ketamir-2
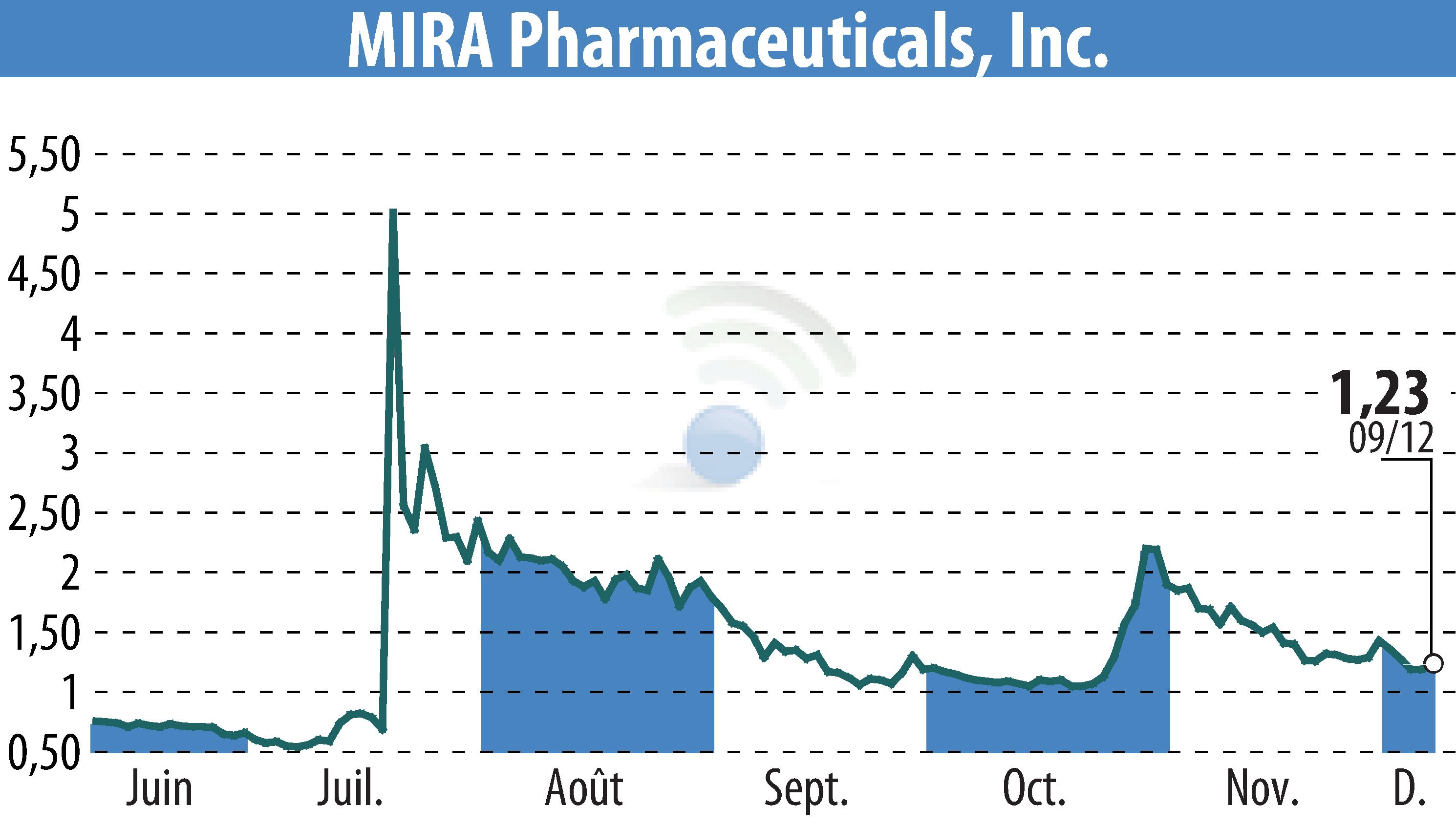
MIRA Pharmaceuticals, Inc. has successfully completed its preclinical safety program for Ketamir-2, a new oral ketamine analog. Validation of its safety profile marks a significant milestone, as the company prepares to submit its Investigational New Drug (IND) application by the end of 2024.
The absence of adverse findings positions MIRA to advance into clinical trials, focusing on neuropathic pain. This development is crucial as nearly 40% of drug failures result from safety issues. Comprehensive studies demonstrated Ketamir-2's safety across various assessments, including cardiovascular and CNS evaluations.
Phase I trials, beginning in early 2025, aim to assess safety, tolerability, and pharmacokinetics in healthy participants. MIRA anticipates starting Phase IIa trials later that year, aligning with its strategic plan for growth and partnership.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MIRA Pharmaceuticals news
