on Moderna, Inc. (NASDAQ:MRNA)
Moderna Gains European Approval for New COVID-19 Vaccine
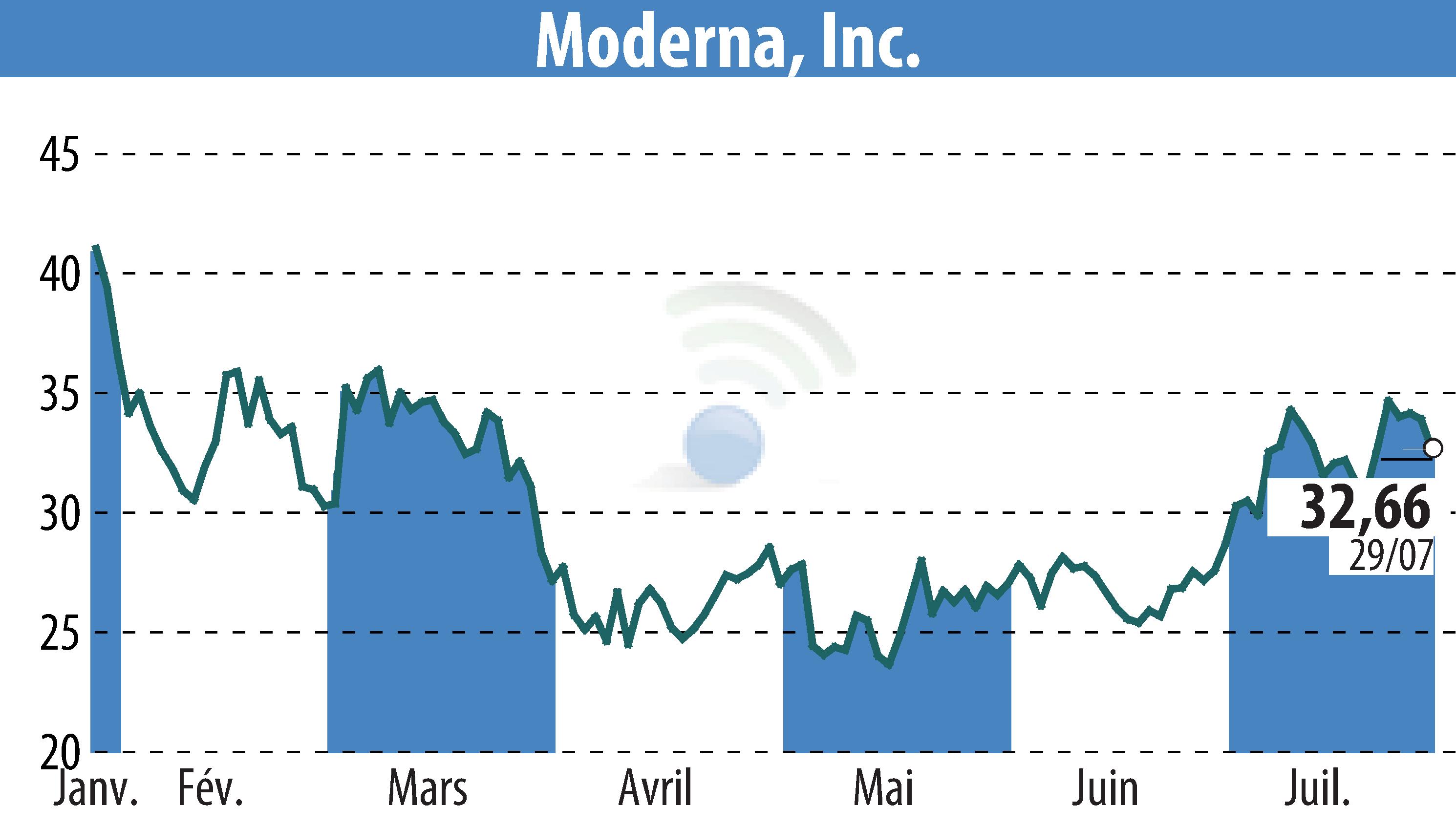
Moderna, Inc. has received approval from the European Commission for its updated COVID-19 vaccine, Spikevax®, targeting the SARS-CoV-2 variant LP.8.1. This authorization is relevant for individuals aged six months and older, encompassing all 27 EU member states, Iceland, Liechtenstein, and Norway.
The approval follows a positive recommendation from the European Medicines Agency's Committee for Medicinal Products for Human Use. Moderna plans to distribute doses in time for the 2025-2026 vaccination season, aligning with guidance from global health authorities recommending updates to address circulating strains.
Moderna's vaccine has generally been well-tolerated, with common adverse reactions including pain at the injection site, headache, fatigue, and chills. Additional regulatory reviews are ongoing worldwide.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
