on Moderna, Inc. (NASDAQ:MRNA)
Moderna's New COVID-19 Vaccine Formula Shows Promising Results
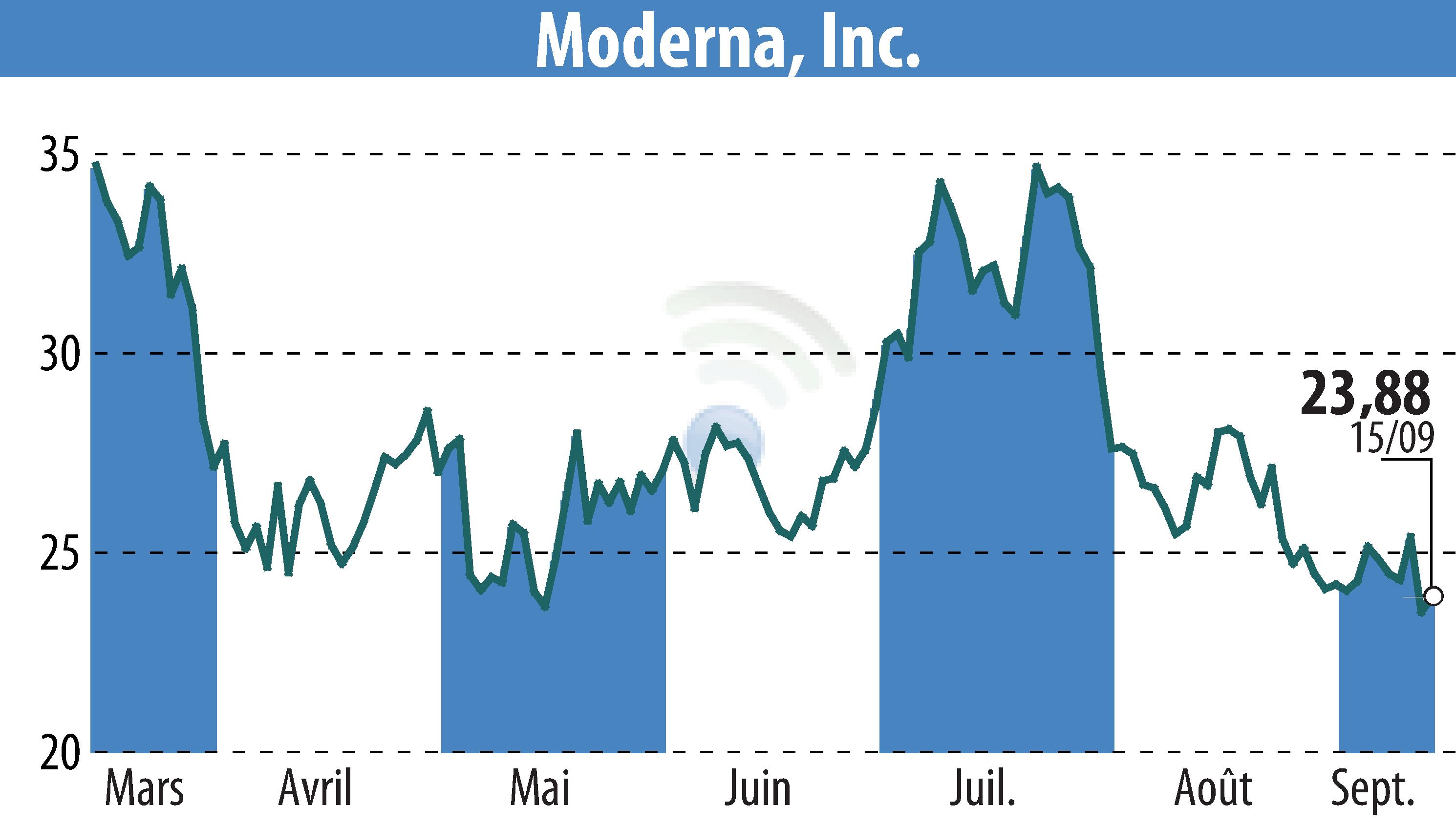
Moderna, Inc. has reported promising preliminary data for its updated COVID-19 vaccine targeting the LP.8.1 variant. The 2025-2026 Spikevax formula demonstrated an over 8-fold increase in neutralizing antibodies across various age groups, according to a Phase 4 clinical trial. This includes individuals aged 12-64 with high-risk conditions and those aged 65 and above.
The new formula aligns with current U.S. viral strains, as confirmed by CDC data. The FDA recently approved this version for high-risk individuals and older adults. Safety profiles remain consistent with previous studies, showing no new safety issues.
Globally, regulators in Canada, Europe, Japan, and other regions have also approved Spikevax’s updated formula, supporting its widespread use.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
