on Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives European Commission Approval for RSV Vaccine mRESVIA(R)
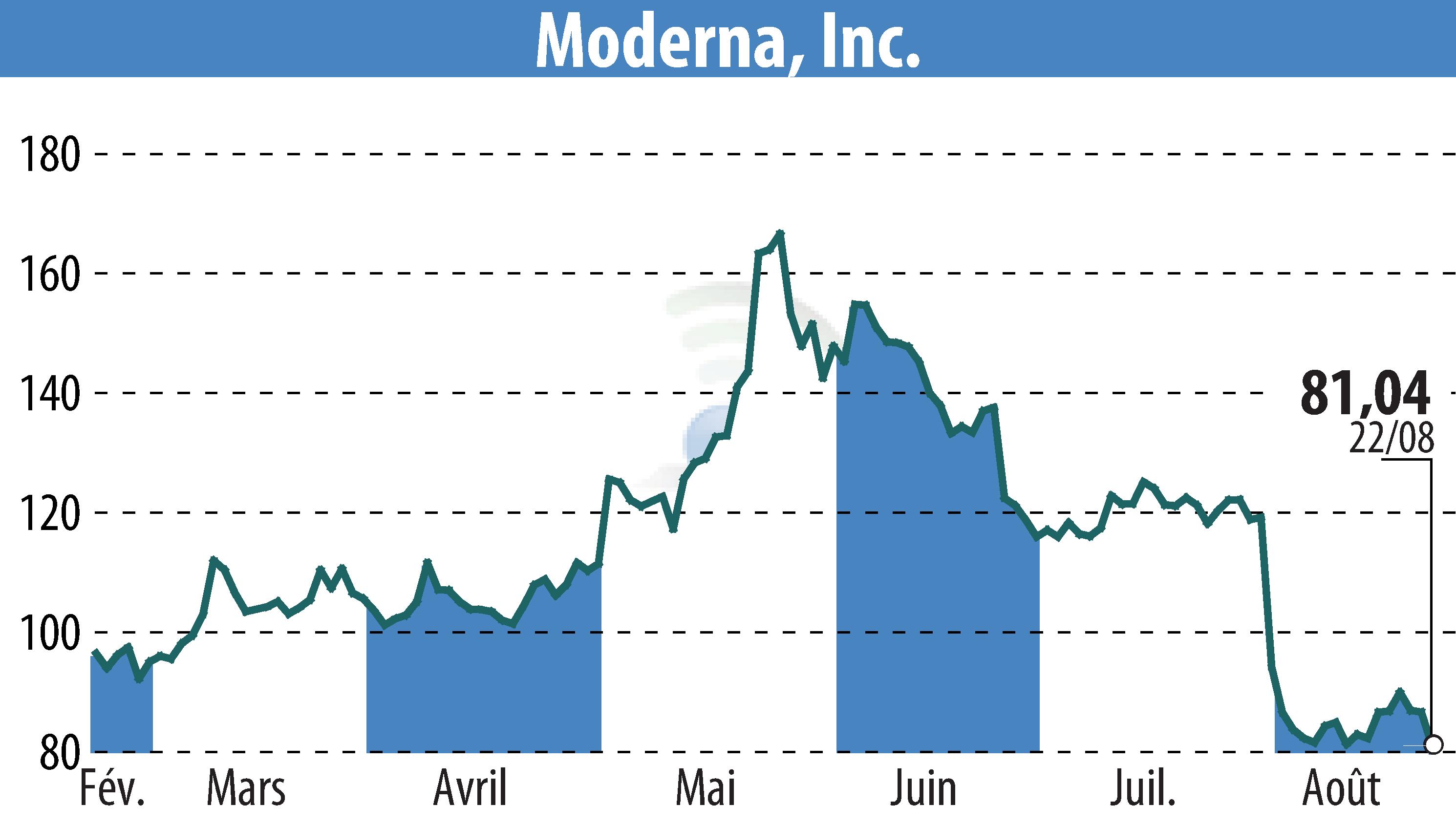
Cambridge, MA / ACCESSWIRE / August 23, 2024 / Moderna, Inc. (NASDAQ:MRNA) today announced that the European Commission (EC) has granted marketing authorization for mRESVIA® (mRNA-1345). The vaccine aims to protect adults aged 60 years and older from lower respiratory tract disease caused by RSV infection. This authorization follows a Positive Opinion from the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP). The approval is valid across all 27 EU member states, Iceland, Liechtenstein, and Norway.
Stéphane Bancel, CEO of Moderna, highlighted the significance of this approval: "This approval marks the first time an mRNA vaccine has been approved for a disease beyond COVID-19 in Europe." mRESVIA, offered in a pre-filled syringe, aims to reduce vaccine preparation time and administrative errors.
The marketing authorization is based on data from the Phase 3 clinical trial ConquerRSV. Conducted in about 37,000 adults aged 60 years or older across 22 countries, the trial found a vaccine efficacy of 83.7% against RSV lower respiratory tract disease. Follow-up analyses confirmed sustained efficacy.
In May 2024, mRESVIA also received approval from the U.S. FDA, under a breakthrough therapy designation, marking Moderna's second approved mRNA product. The company has filed for marketing authorization in multiple markets worldwide.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
