on Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives UK Approval for RSV Vaccine mRESVIA®
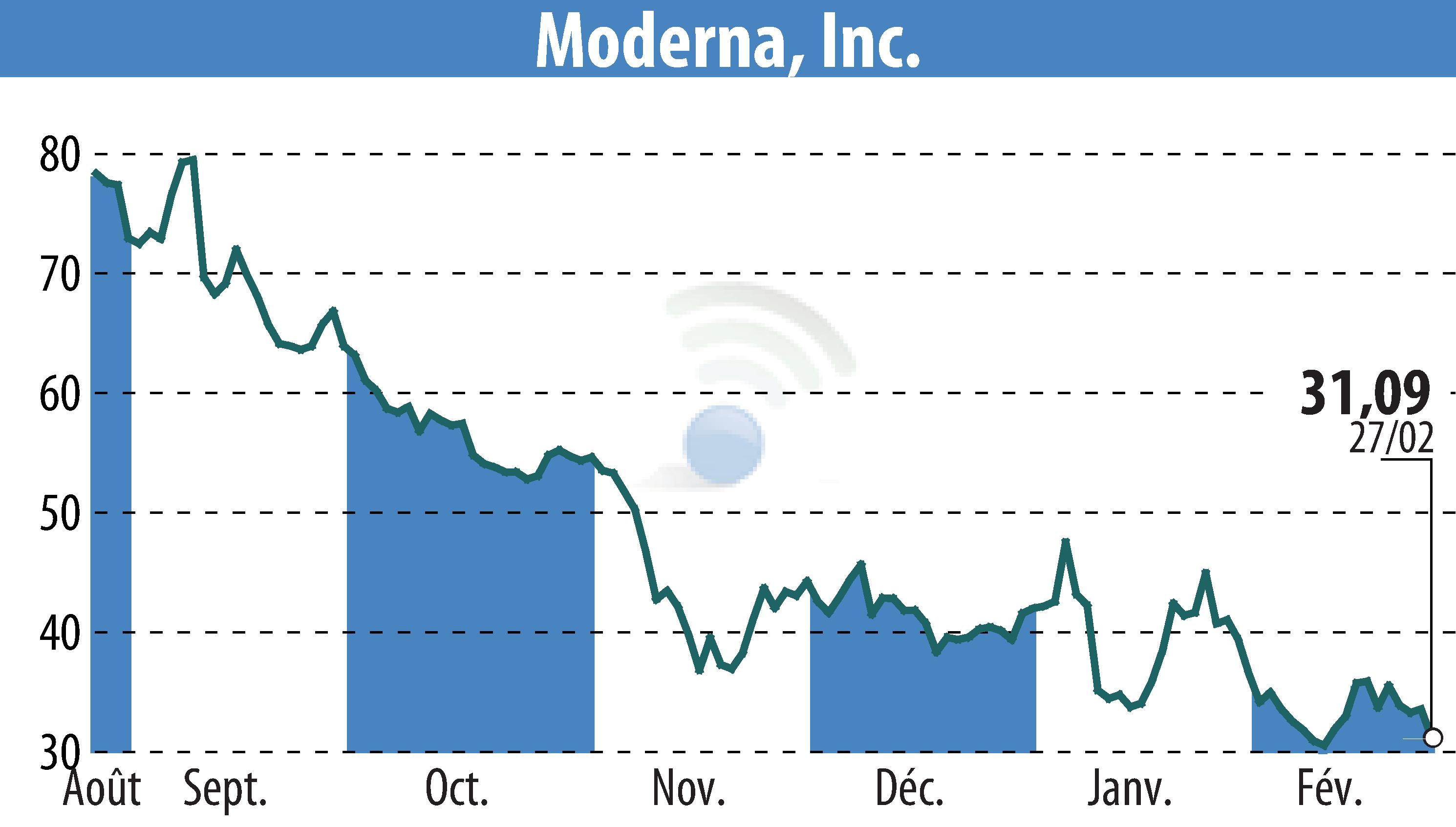
Moderna, Inc. has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK has granted marketing authorization for its RSV vaccine, mRESVIA® (mRNA-1345), aimed at preventing lower respiratory tract disease in adults aged 60 and over. This marks Moderna's second approved product in the UK, signaling a significant step toward enhancing respiratory disease preparedness.
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory infections and pneumonia among the elderly in the UK, contributing to numerous hospitalizations and deaths annually. The approval follows positive outcomes from the Phase 3 ConquerRSV trial, involving 37,000 participants worldwide, and showed no major safety issues.
Moderna's Oxfordshire site will manufacture the vaccine. This UK approval joins previous authorizations in the US, EU, Canada, and other regions. Moderna continues to seek regulatory approvals globally.
R. E.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
