on Moderna, Inc. (NASDAQ:MRNA)
Moderna Secures Health Canada Approval for RSV Vaccine for Seniors
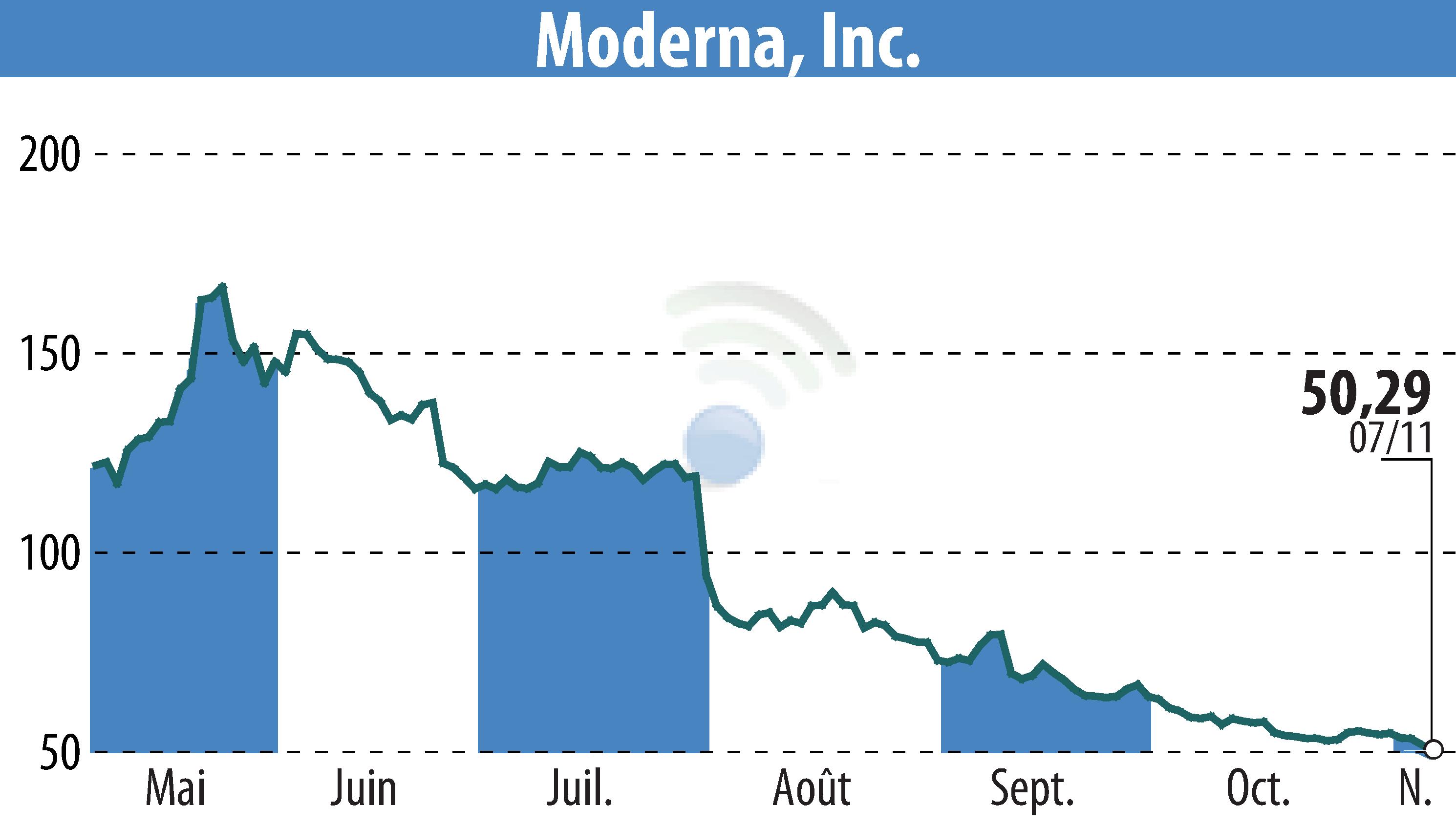
Moderna, Inc. has received approval from Health Canada for its mRESVIA™ vaccine. This marks Canada's first mRNA vaccine targeting the respiratory syncytial virus (RSV) for individuals aged 60 and older. mRESVIA™ is Moderna's second approved product in Canada, positioning the country as the fourth to authorize its use, after the U.S., Europe, and Qatar. The vaccine is uniquely available in pre-filled syringes, simplifying administration and minimizing errors.
The National Advisory Committee on Immunization recommends RSV vaccination for Canadians aged 75 and older, as well as residents of nursing homes. Community-dwelling seniors aged 60 and above are advised to consult healthcare providers for vaccination decisions. Moderna anticipates mRESVIA's availability in Canada by early 2025.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
