on Moderna, Inc. (NASDAQ:MRNA)
Moderna's mNEXSPIKE COVID-19 Vaccine Secures FDA Approval
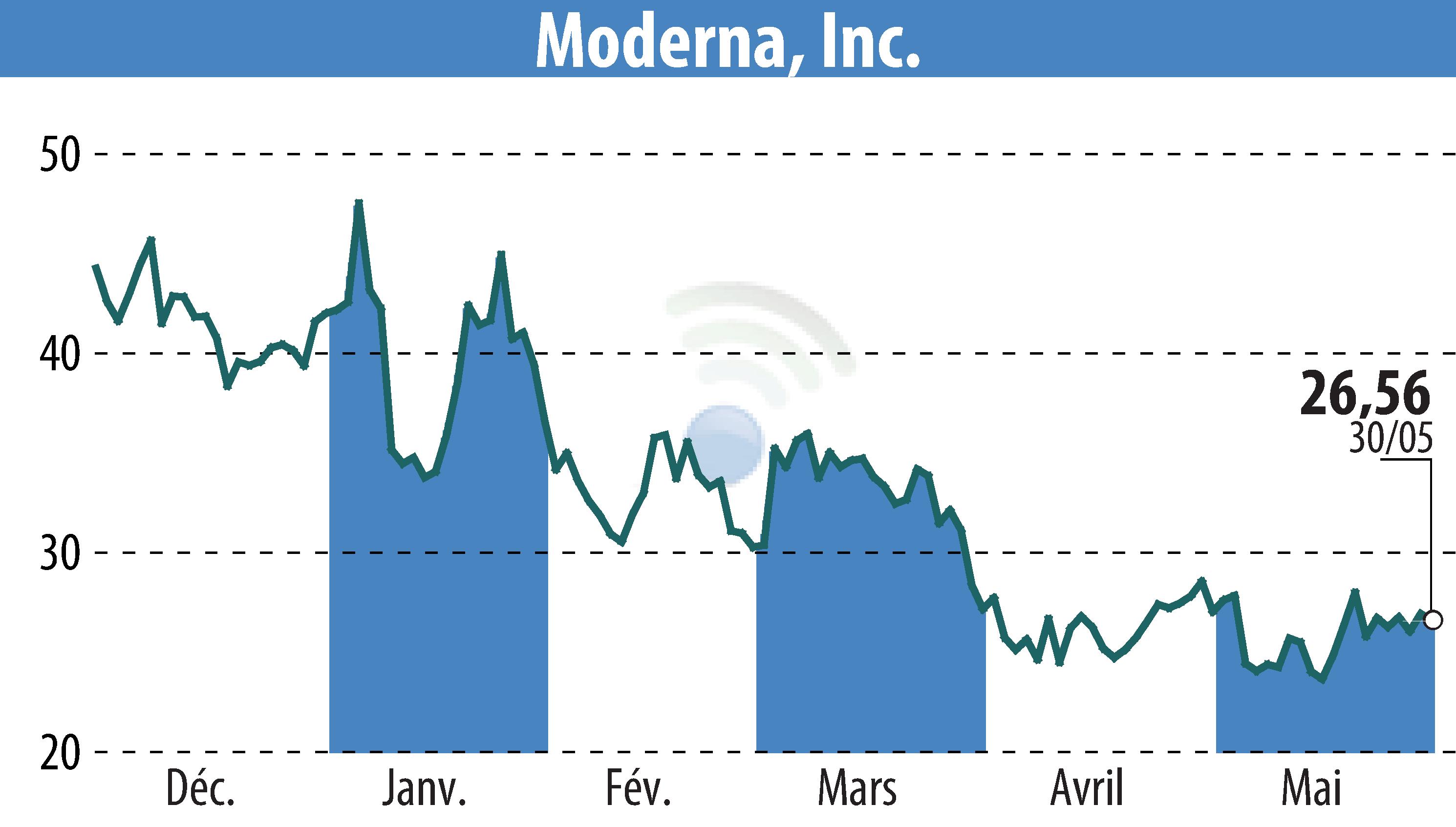
Moderna, Inc. has announced that the U.S. FDA has approved its third product, mNEXSPIKE, a COVID-19 vaccine designed for adults 65 and older and individuals aged 12 to 64 with underlying health risks. This approval aims to offer enhanced protection against severe COVID-19 cases.
The decision by the FDA stems from a Phase 3 clinical trial involving about 11,400 participants. The trial demonstrated that mNEXSPIKE has a 9.3% higher vaccine efficacy compared to Moderna's Spikevax in those 12 and older, and a 13.5% higher efficacy in those 65 and older.
Moderna plans to make mNEXSPIKE available for the 2025-2026 respiratory virus season in the U.S. The vaccine shares a similar safety profile with Spikevax, showing fewer local reactions and similar systemic responses.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Moderna, Inc. news
