on NANOBIOTIX (EPA:NANO)
NANOBIOTIX Reveals Promising Phase 1 Study Results for Esophageal Cancer Treatment
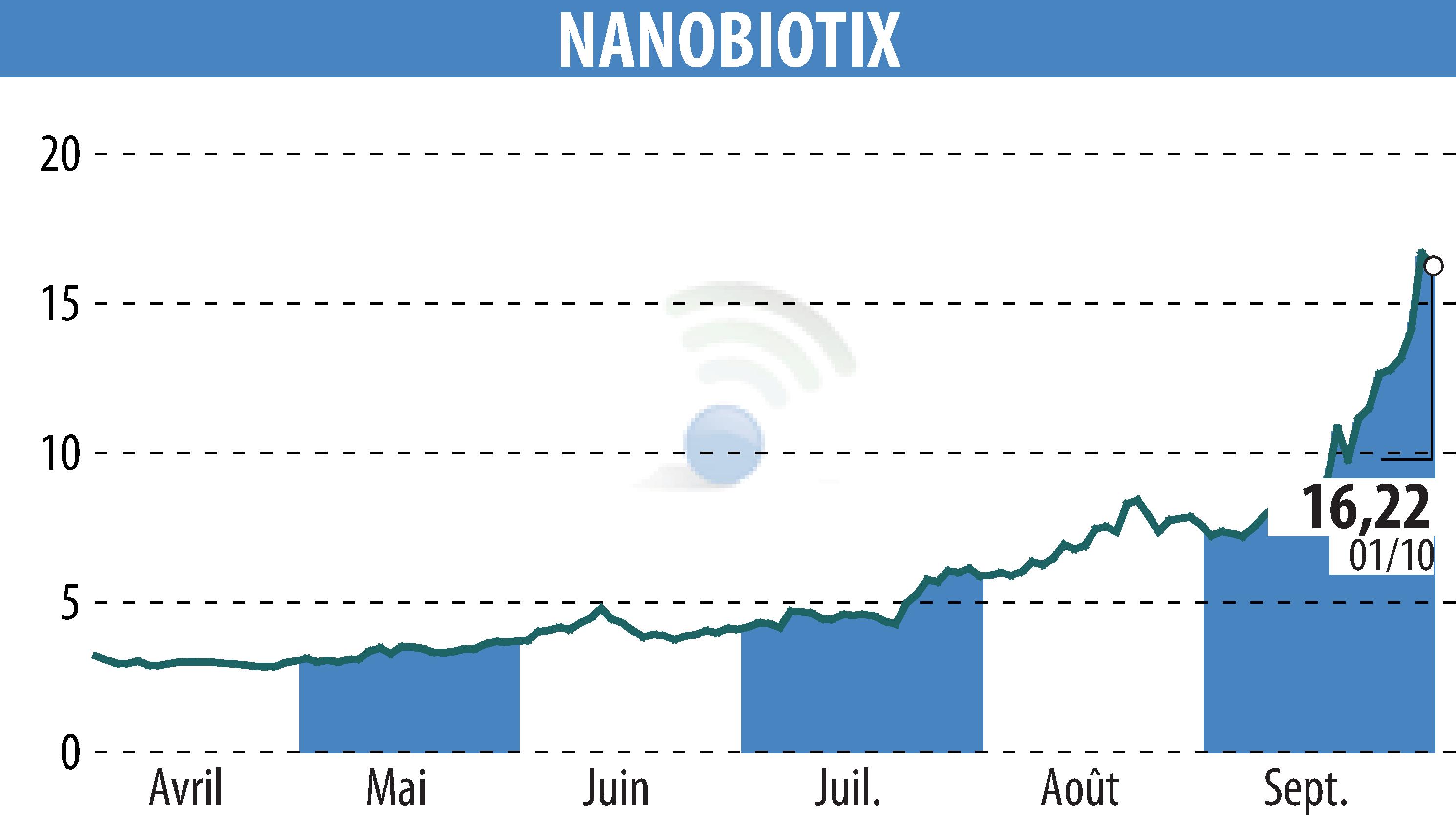
NANOBIOTIX has released encouraging data from its Phase 1 study of JNJ-1900 (NBTXR3) for esophageal cancer. The treatment was well-tolerated in 13 patients. Injection feasibility has been confirmed for those with locally advanced adenocarcinoma of the esophagus. The study reported an 85% disease control rate (DCR) and a 69% objective response rate (ORR), including complete and partial responses.
Conducted by MD Anderson Cancer Center, this study also explored the combination of JNJ-1900 (NBTXR3) with photon or proton chemoradiation. Recruitment of additional patients is ongoing. The promising results support the potential of JNJ-1900 (NBTXR3) to improve tumor control and potentially reduce the need for invasive surgeries.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NANOBIOTIX news
