on NanoViricides, Inc. (NASDAQ:NNVC)
NanoViricides Advances NV-387 to Phase II Trials for Multiple Viral Infections
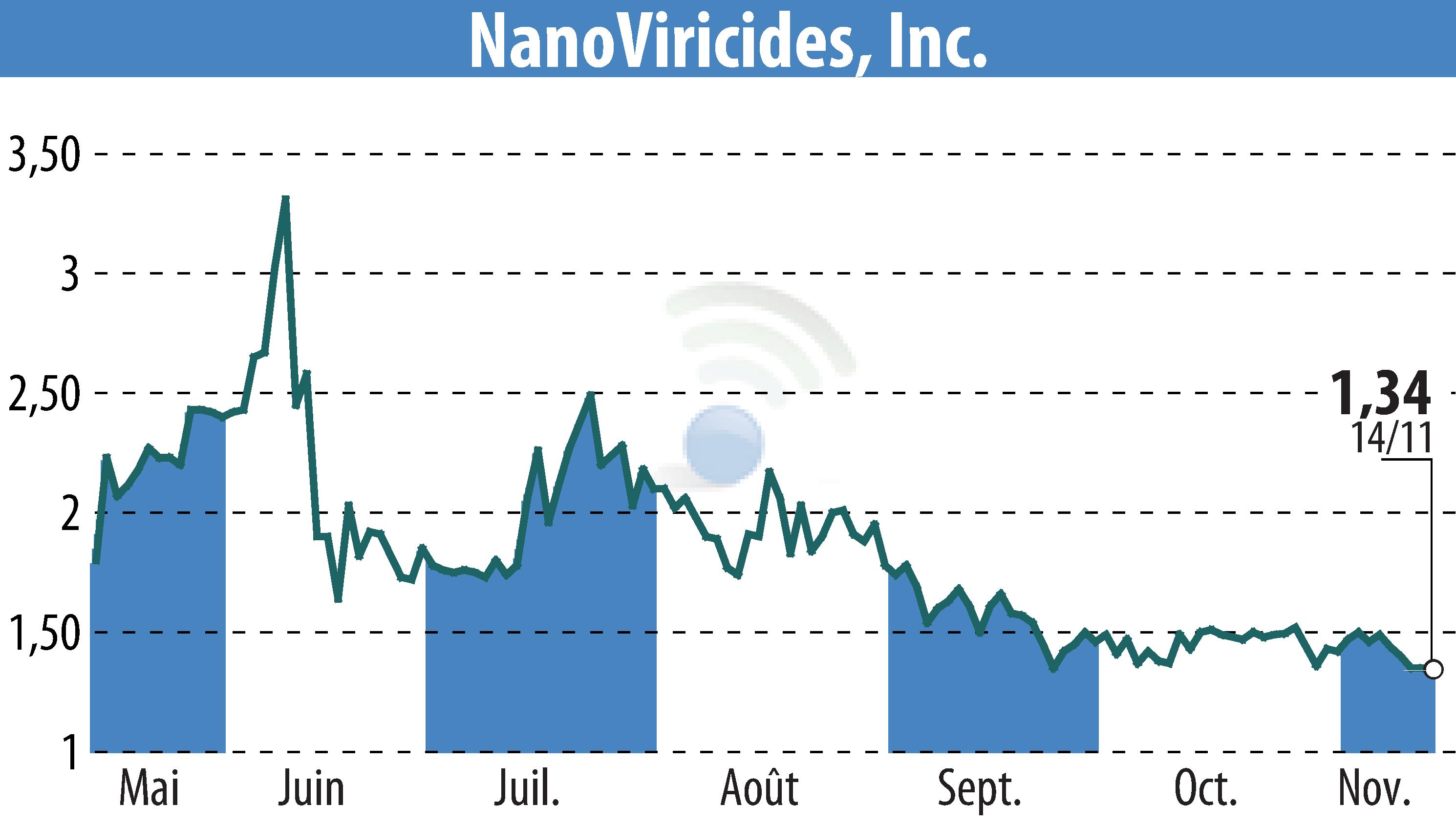
NanoViricides, Inc. has filed its quarterly report and announced progress in developing NV-387, a broad-spectrum antiviral drug. NV-387 is moving toward Phase II clinical trials to treat MPOX infection in Central Africa and RSV in the USA. The drug has shown promising activity against viruses like COVID, RSV, Influenza, and MPOX/Smallpox. Financially, the company reports cash assets of $3.87 million with liabilities of $1.63 million as of September 30, 2024. Funding efforts raised approximately $1.71 million through an At-the-Market offering. Nevertheless, NanoViricides indicates a shortfall in funding to cover planned operations through November 2025.
The company highlights the unique mechanism of NV-387, which targets virus particles by mimicking cell entry pathways, potentially preventing virus escape. The regulatory strategy involves advancing to Phase II trials, seeking partnerships, and non-dilutive funding to continue development. While NV-387’s capability shows promise, the typical path to drug approval requires significant resources and time, emphasizing the inherent uncertainties in drug development.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NanoViricides, Inc. news
