on NanoViricides, Inc. (NASDAQ:NNVC)
NanoViricides Updates Clinical Program and Strategy
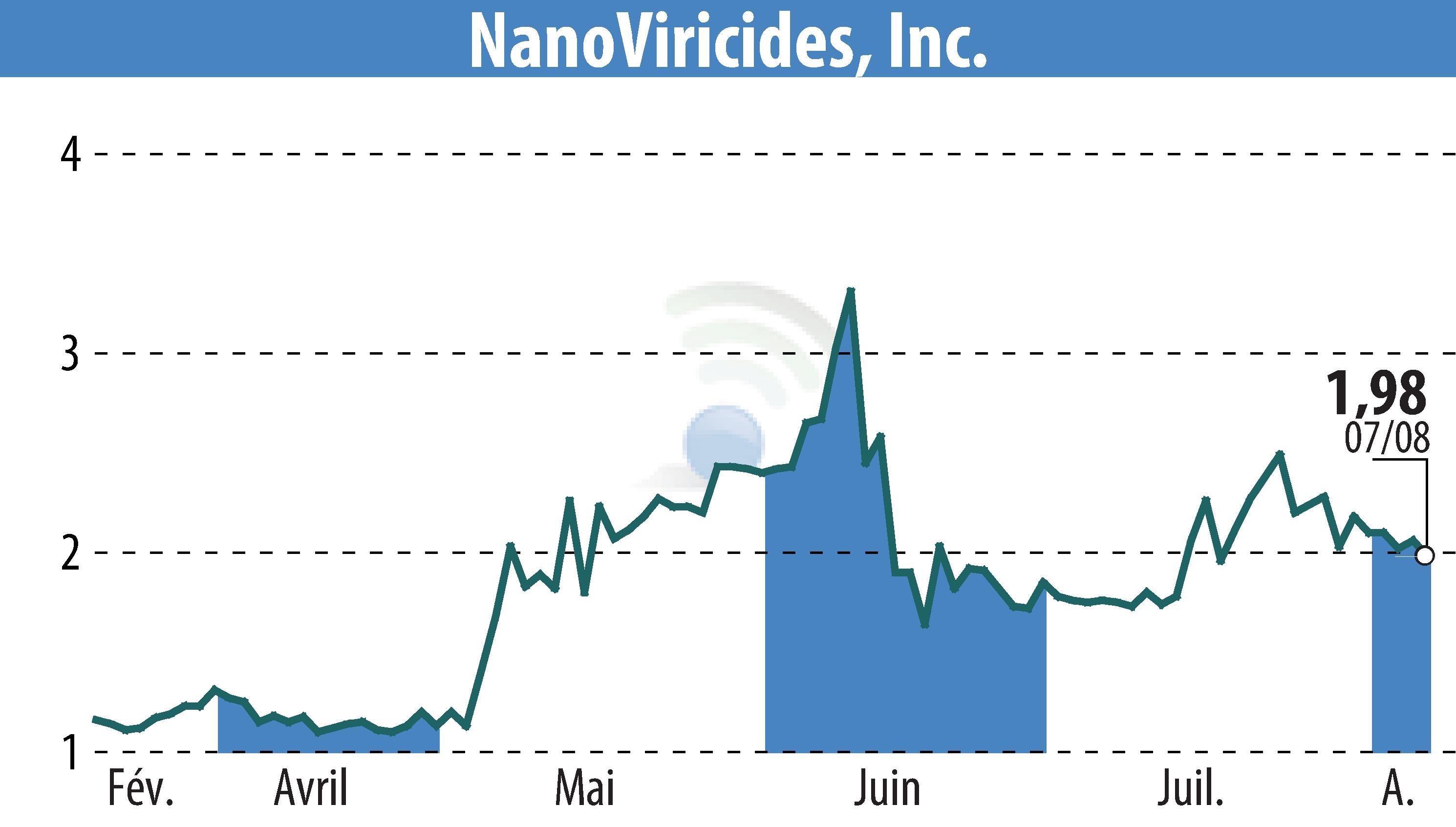
NanoViricides, Inc. (NYSE American: NNVC), a leader in broad-spectrum antiviral nanomedicines, announced an update on its NV-387 clinical program and strategy. NV-387 is their first clinical stage drug designed to mimic sulfated-proteoglycan features that many human viruses use to infect cells. Animal trials showed NV-387’s effectiveness against Influenza, COVID, RSV, and orthopoxviruses like Smallpox.
The company reported that NV-387 provided complete protection in an RSV study on lethally lung-infected mice, outperforming the current treatment, Ribavirin. This broad-spectrum efficacy suggests that viruses are unlikely to develop resistance against NV-387, unlike current medical countermeasures.
NanoViricides has completed a database audit of its Phase 1a/1b human clinical trial in India and plans to commence Phase II trials to evaluate NV-387’s effectiveness against multiple respiratory viruses under a novel adaptive trial design. Additionally, they aim to seek regulatory approvals for treating pediatric RSV disease and secure big pharma collaborations to expedite their programs.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all NanoViricides, Inc. news
