on VALNEVA (EPA:VLA)
Partial suspension of the use of Valneva's IXCHIQ® vaccine
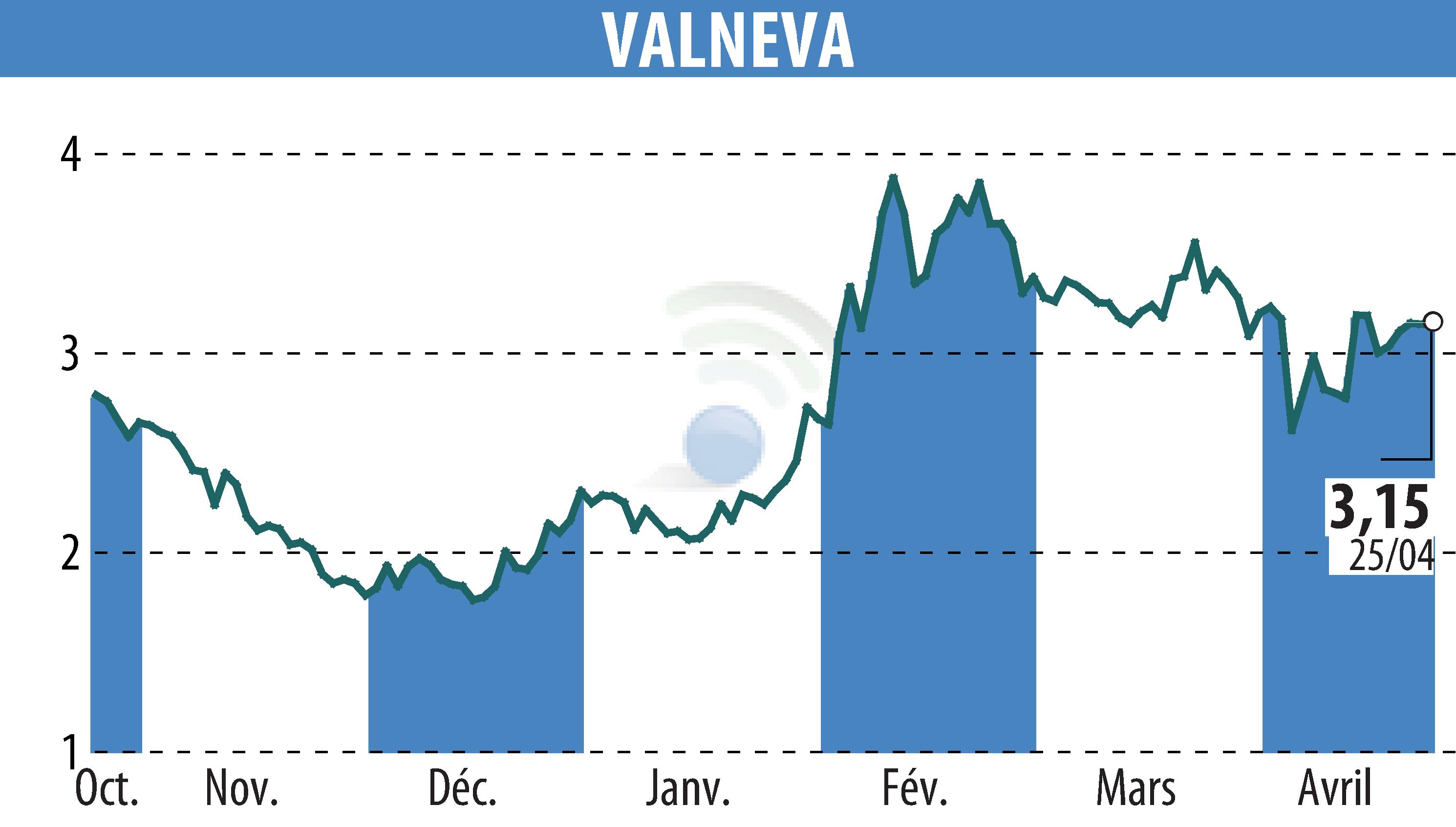
On April 26, 2025, Valneva SE announced an updated recommendation by the French High Authority for Health regarding the IXCHIQ® chikungunya vaccine. This comes after reports of serious adverse events in people over 80 years of age with comorbidities during the campaign in Réunion and Mayotte.
In response, French authorities have decided to suspend the use of IXCHIQ® for people aged 65 and over until further information becomes available. Notably, three serious cases of hospitalization have been reported, including one death. For the time being, the vaccine remains recommended for those aged 18-64.
Valneva is working closely with authorities to determine next steps and continue to ensure the safety of the vaccine. 40,000 doses have already been delivered to Réunion Island during the ongoing outbreak.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
