on Nanohale AG (isin : DE000A1EWVY8)
Positive CHMP Opinion for Formycon's Aflibercept-Biosimilar FYB203
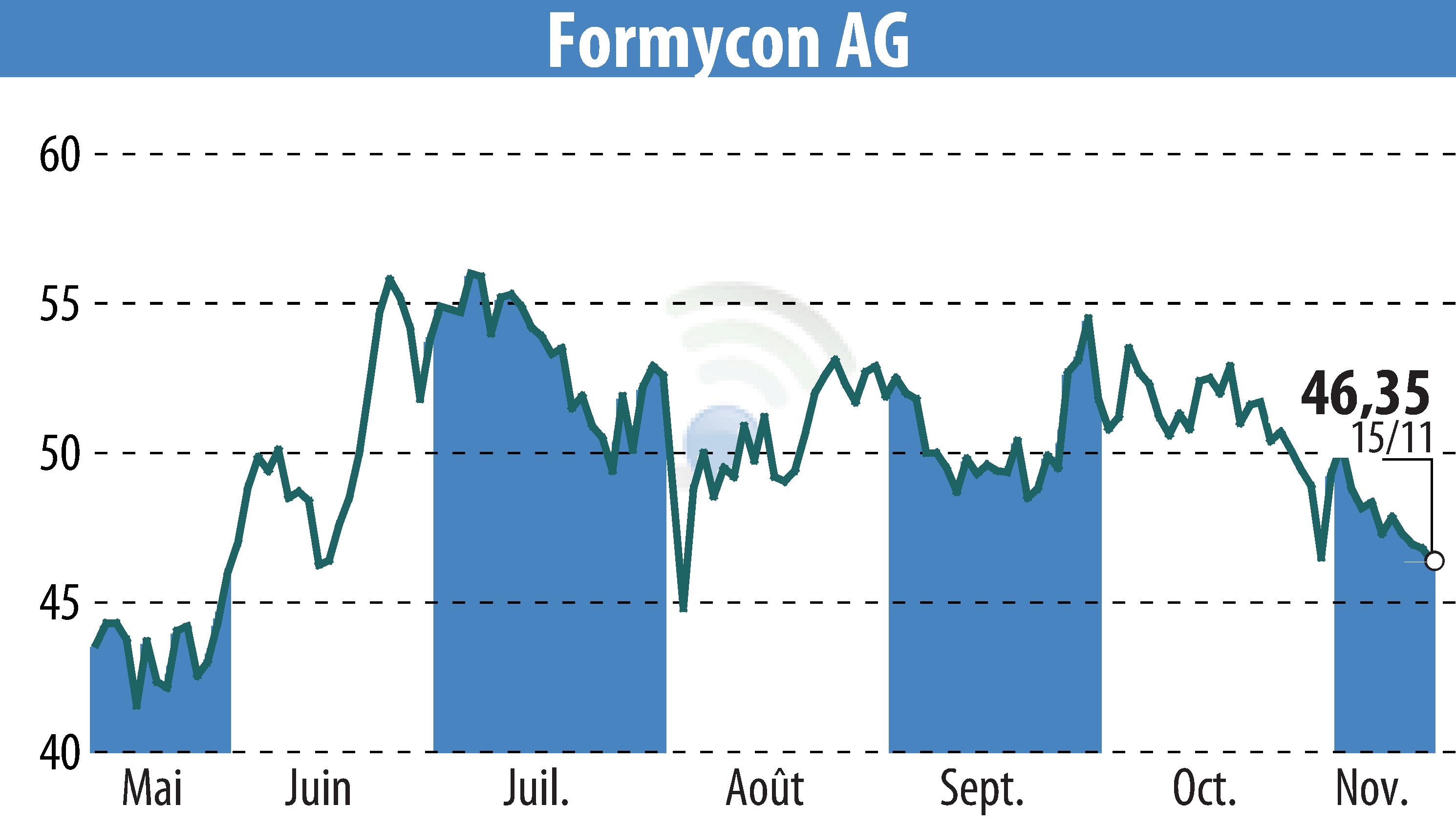
Formycon AG has announced that the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has granted a positive opinion for FYB203, their aflibercept-biosimilar, known as AHZANTIVE®/ Baiama®. This recommendation is a crucial step towards obtaining European Union market approval for the treatment of Age-Related Neovascular Macular Degeneration (nAMD) and other retinal diseases.
The CHMP's endorsement follows a comprehensive review of analytical, pre-clinical, clinical, and manufacturing data. FYB203 showcased equivalent quality, efficacy, and safety to the reference product, Eylea®. The European Commission's marketing authorization is anticipated by January 2025.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Nanohale AG news
