on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Seeks EMA Approval for Icotrokinra in Psoriasis
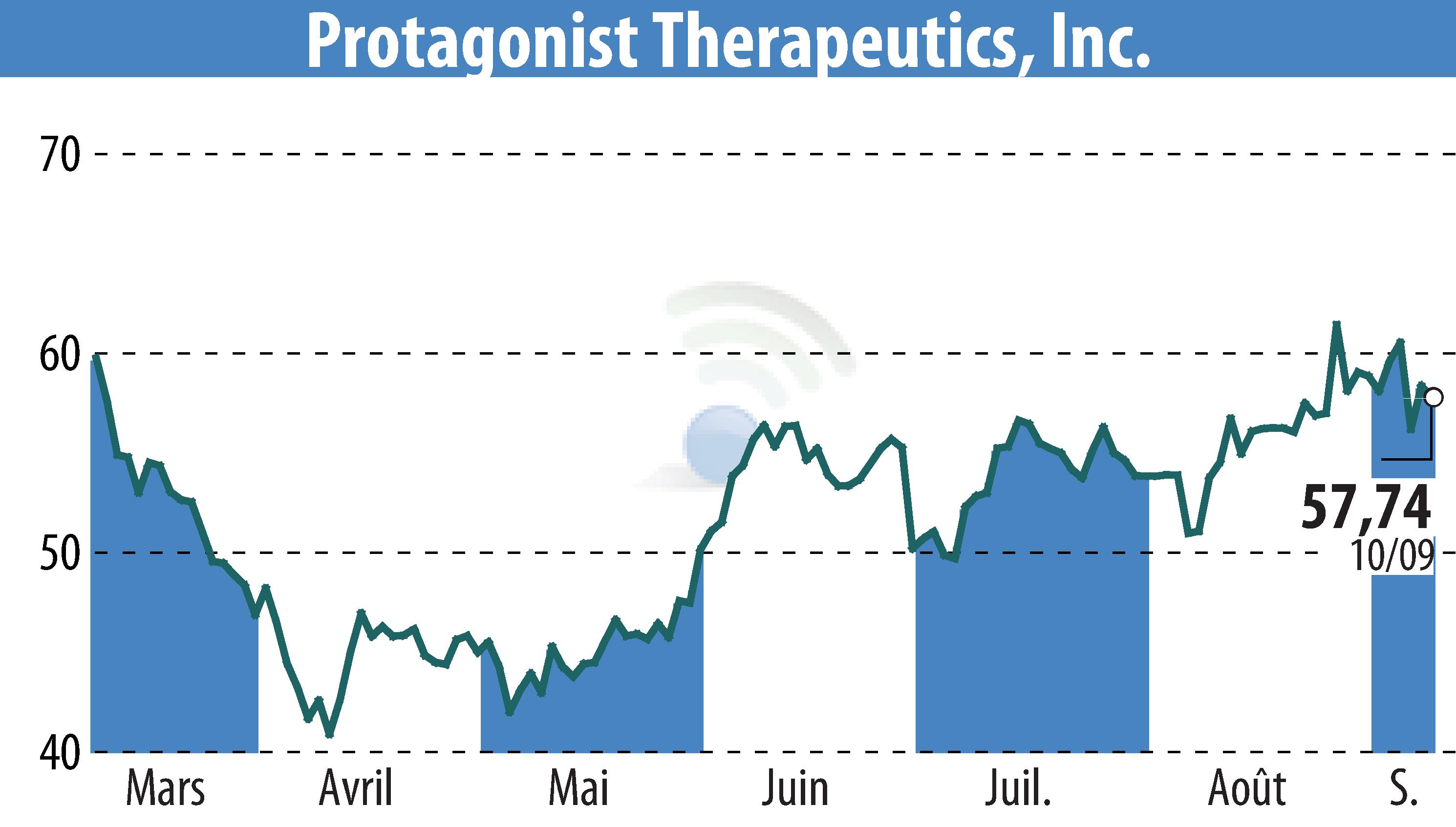
Protagonist Therapeutics has submitted an application to the European Medicines Agency for icotrokinra's approval, a first-in-class oral peptide targeting the IL-23 receptor, vital for plaque psoriasis treatment. This application is backed by four Phase 3 studies, demonstrating significant efficacy and safety, including head-to-head superiority against deucravacitinib and prevalent with both adult and adolescent patients.
Icotrokinra demonstrated superior outcomes in moderate-to-severe plaque psoriasis, emphasizing complete skin clearance in difficult-to-treat areas. The once-daily tablet offers patients a favorable safety profile. The submission aligns with an earlier NDA filed with the U.S. FDA, showcasing icotrokinra's potential to enhance psoriasis care paradigms.
The ICONIC program's comprehensive data underline the potential of icotrokinra in disrupting current treatment approaches, with further studies underway addressing additional conditions like ulcerative colitis.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
