on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics Presents Novel Findings on Icotrokinra and PN-881 at EADV 2025
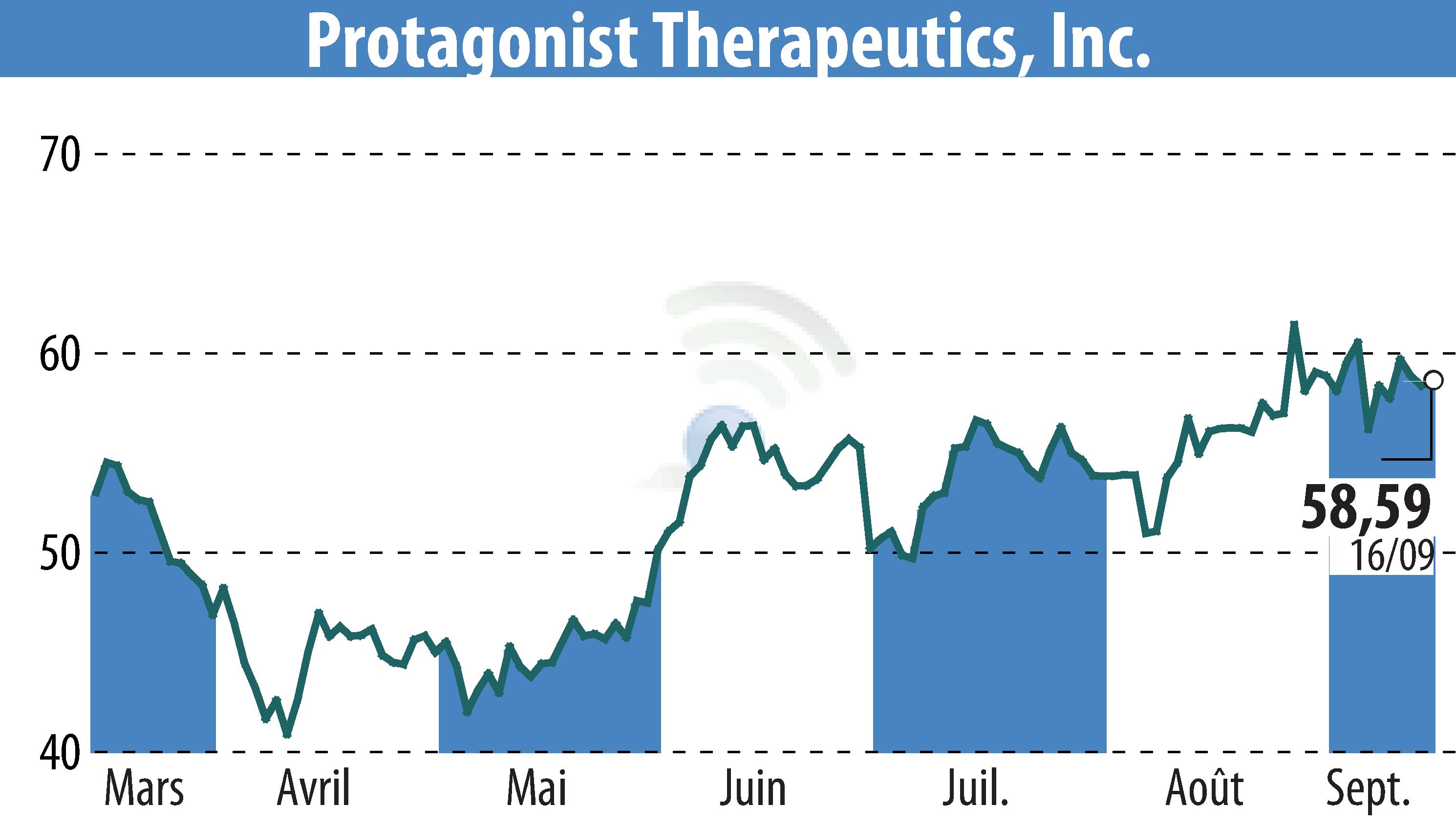
Protagonist Therapeutics recently shared significant advancements in its clinical and preclinical studies at the EADV 2025 Congress. The company's investigational oral peptide, Icotrokinra, exhibited enhanced skin clearance in psoriasis patients compared to deucravacitinib, maintaining safety akin to placebo in the Phase 3 ICONIC-ADVANCE studies. Furthermore, long-term data from the Phase 3 ICONIC-LEAD study indicated sustained efficacy and safety at 52 weeks.
The preclinical study on PN-881, a novel oral peptide targeting the IL-17 pathway, showed promising results in metabolic stability and potency, marking a step towards initiating Phase 1 trials. Protagonist aims to innovate psoriasis treatment with these oral therapies, potentially setting new standards in the field.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
