on Protagonist Therapeutics, Inc. (NASDAQ:PTGX)
Protagonist Therapeutics' Rusfertide Receives FDA Breakthrough Therapy Designation
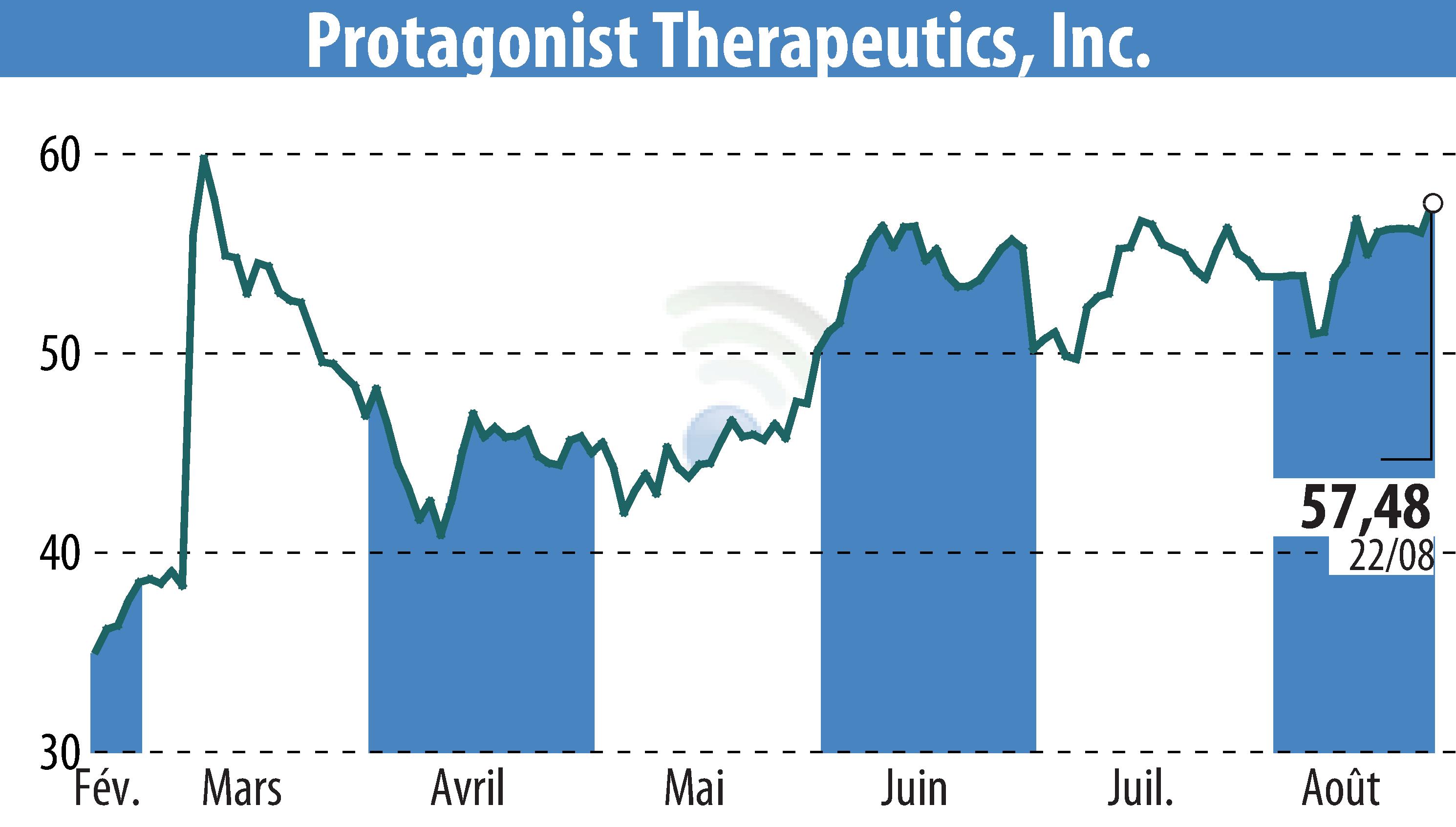
Protagonist Therapeutics announced that the U.S. FDA has granted Breakthrough Therapy Designation to rusfertide for erythrocytosis treatment in polycythemia vera (PV) patients. This follows its previous Orphan Drug and Fast Track designations, offering multiple developmental advantages. The company plans to submit a New Drug Application in Q4 2025.
The positive 32-week results from Phase 3 VERIFY study supported the breakthrough designation. These findings were highlighted at the 2025 ASCO Annual Meeting. According to CEO Dinesh V. Patel, rusfertide shows potential to surpass current PV therapies. Chief Medical Officer Arturo Molina emphasized its effectiveness in hematocrit control and reducing phlebotomy dependence.
R. H.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all Protagonist Therapeutics, Inc. news
