on SANOFI-AVENTIS (EPA:SAN)
Sanofi's rilzabrutinib designated an orphan drug in the United States
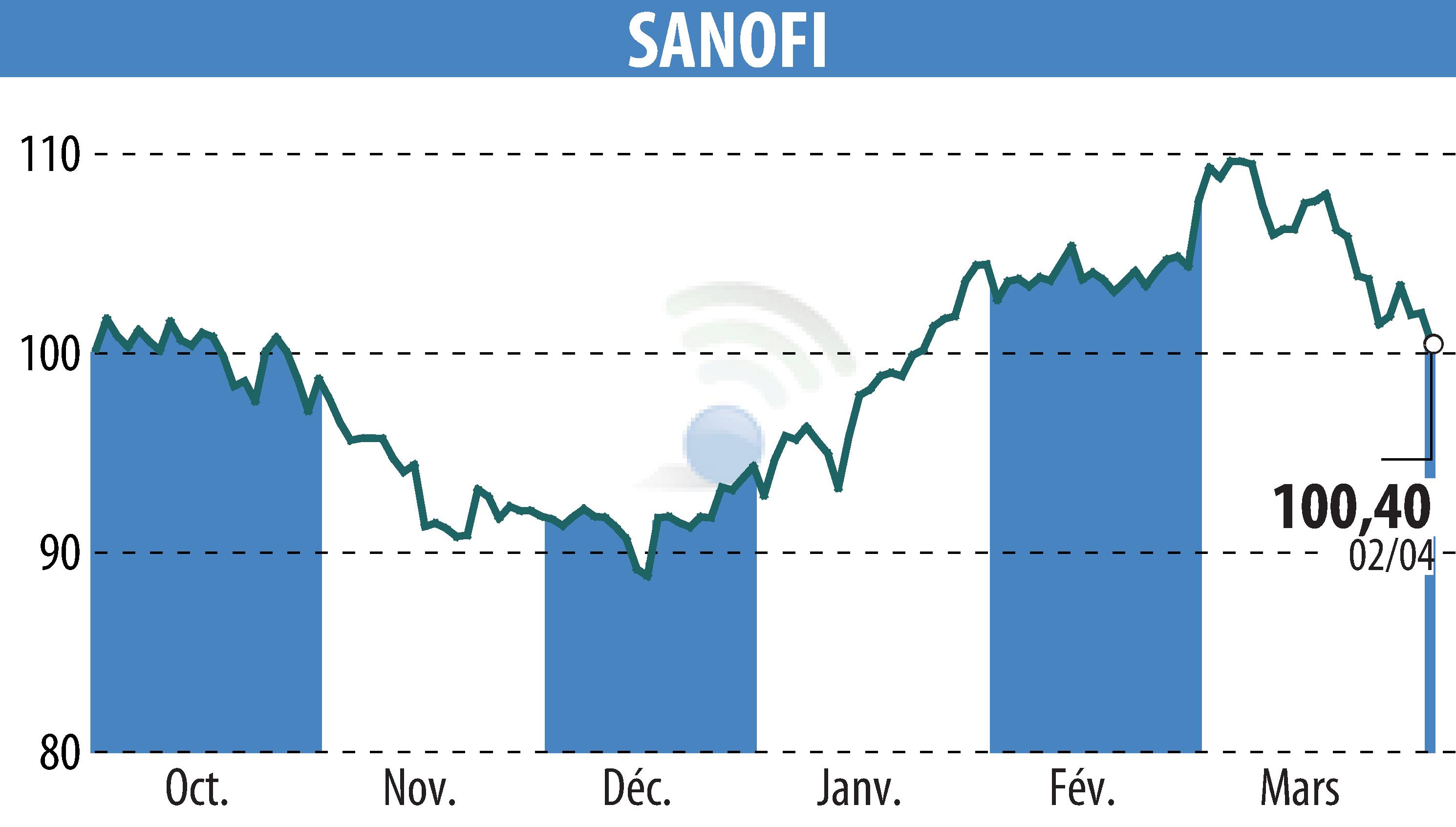
On April 3, 2025, the FDA granted orphan drug designation to Sanofi's rilzabrutinib to treat two rare diseases: warm-antibody hemolytic anemia and IgG4-associated disease, for which no treatments are currently approved. These conditions affect fewer than 200,000 people in the United States.
Rilzabrutinib is currently being studied for immune thrombocytopenia in the Americas, Europe, and China. The FDA is expected to decide on this application in August 2025. Sanofi has already been granted orphan drug designation for this indication in the United States, the EU, and Japan.
Ongoing studies suggest that rilzabrutinib offers promising results in terms of symptom reduction for both diseases. At the same time, the safety profile remains similar to that of previous studies.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
