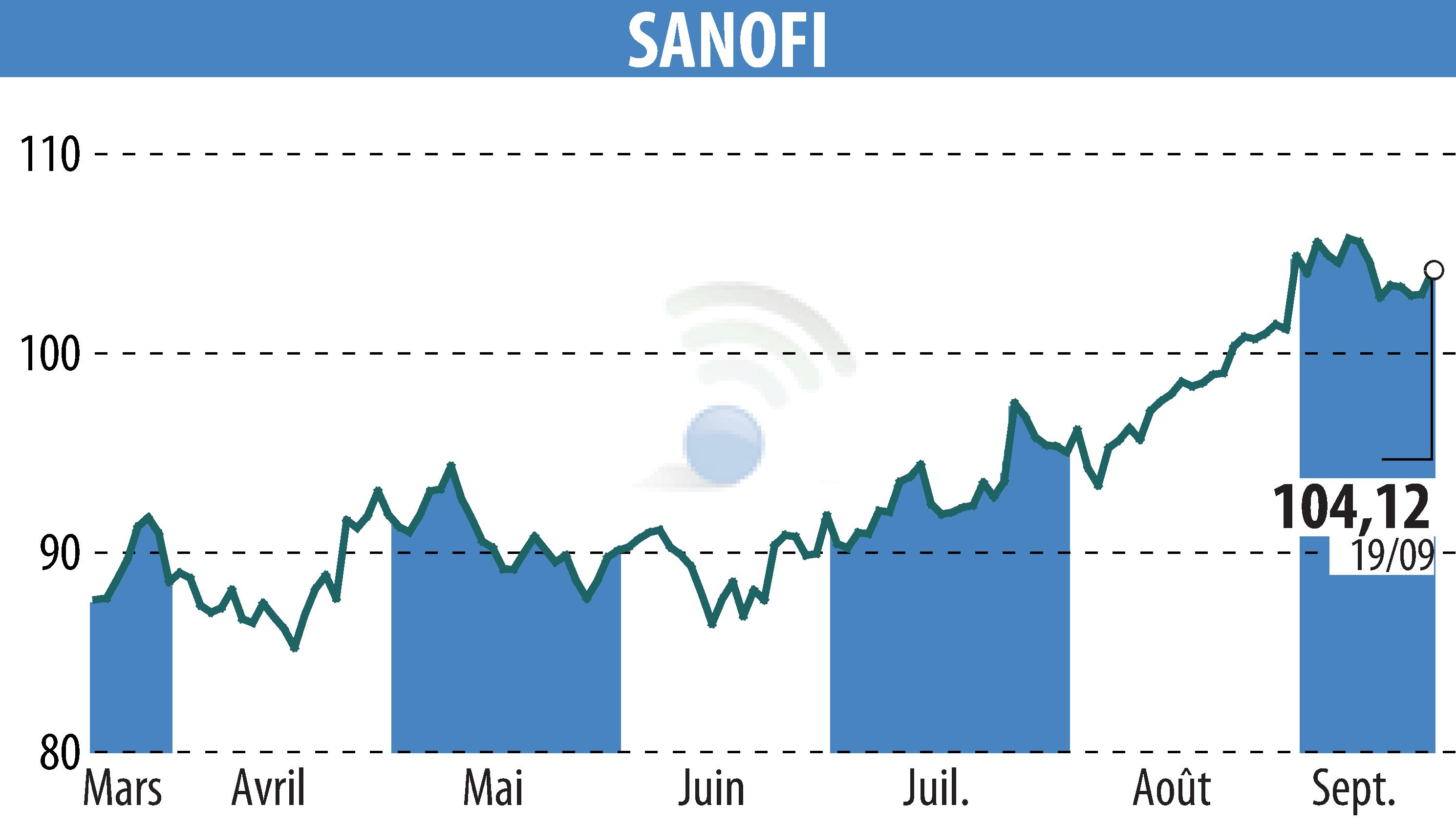
on SANOFI-AVENTIS (EPA:SAN)
Sanofi's Tolebrutinib Shows Promise in Delaying Disability Progression in MS
Sanofi's phase 3 HERCULES study revealed that tolebrutinib delayed the onset of 6-month confirmed disability progression (CDP) by 31% in patients with non-relapsing secondary progressive multiple sclerosis (nrSPMS) compared to a placebo. The findings were presented at the ECTRIMS 2024 conference in Copenhagen.
Secondary analyses highlighted nearly double the rate of confirmed disability improvement in tolebrutinib-treated patients (10%) compared to the placebo group (5%). These positive outcomes are anticipated to spur global regulatory submissions starting in the second half of 2024.
However, the study also reported some adverse events, including elevated liver enzymes in 4.1% of tolebrutinib users versus 1.6% in the placebo group. To address these, Sanofi has implemented more stringent monitoring protocols.
Additional data from the GEMINI 1 and 2 studies showed that tolebrutinib did not surpass Aubagio in improving annualized relapse rates (ARR) for relapsing MS but demonstrated a 29% improvement in delaying disability worsening.
These results collectively suggest that tolebrutinib may effectively manage progression in MS, independent of relapse activity. Further studies and regulatory discussions are anticipated.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
