on SANOFI-AVENTIS (EPA:SAN)
Sarclisa Improves Progression-Free Survival for Multiple Myeloma Patients Eligible for Transplant
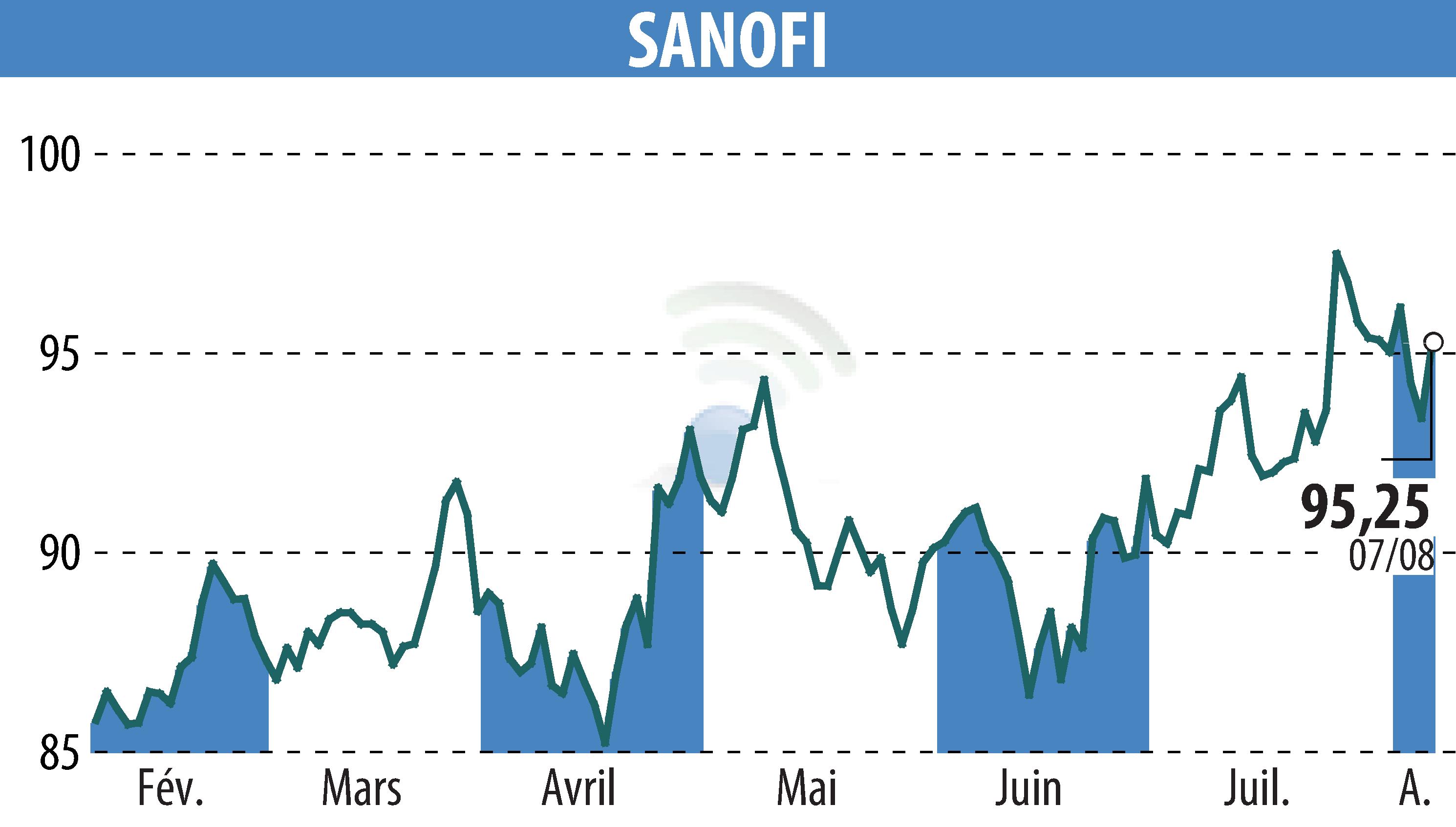
Sanofi-Aventis announced positive results for Sarclisa (isatuximab) in induction treatment with lenalidomide, bortezomib and dexamethasone (RVd). GMMG-HD7 Phase 3 Study Shows Significant Improvement in Progression-Free Survival (PFS) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Eligible for Transplant.
The addition of Sarclisa to standard therapy has demonstrated a notable reduction in disease progression or death. Professor Hartmut Goldschmidt emphasized the importance of this induction therapy in reducing the risk of relapse. The results confirm the potential of Sarclisa as a disease-modifying treatment in this population.
Full results will be presented at an upcoming medical meeting. Sarclisa is already approved in more than 50 countries for the treatment of certain forms of multiple myeloma, with several clinical studies underway to evaluate its effectiveness in various settings.
R. H.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SANOFI-AVENTIS news
