on SENSORION (EPA:ALSEN)
Sensorion Reports First Half 2024 Financial Results and Clinical Advances
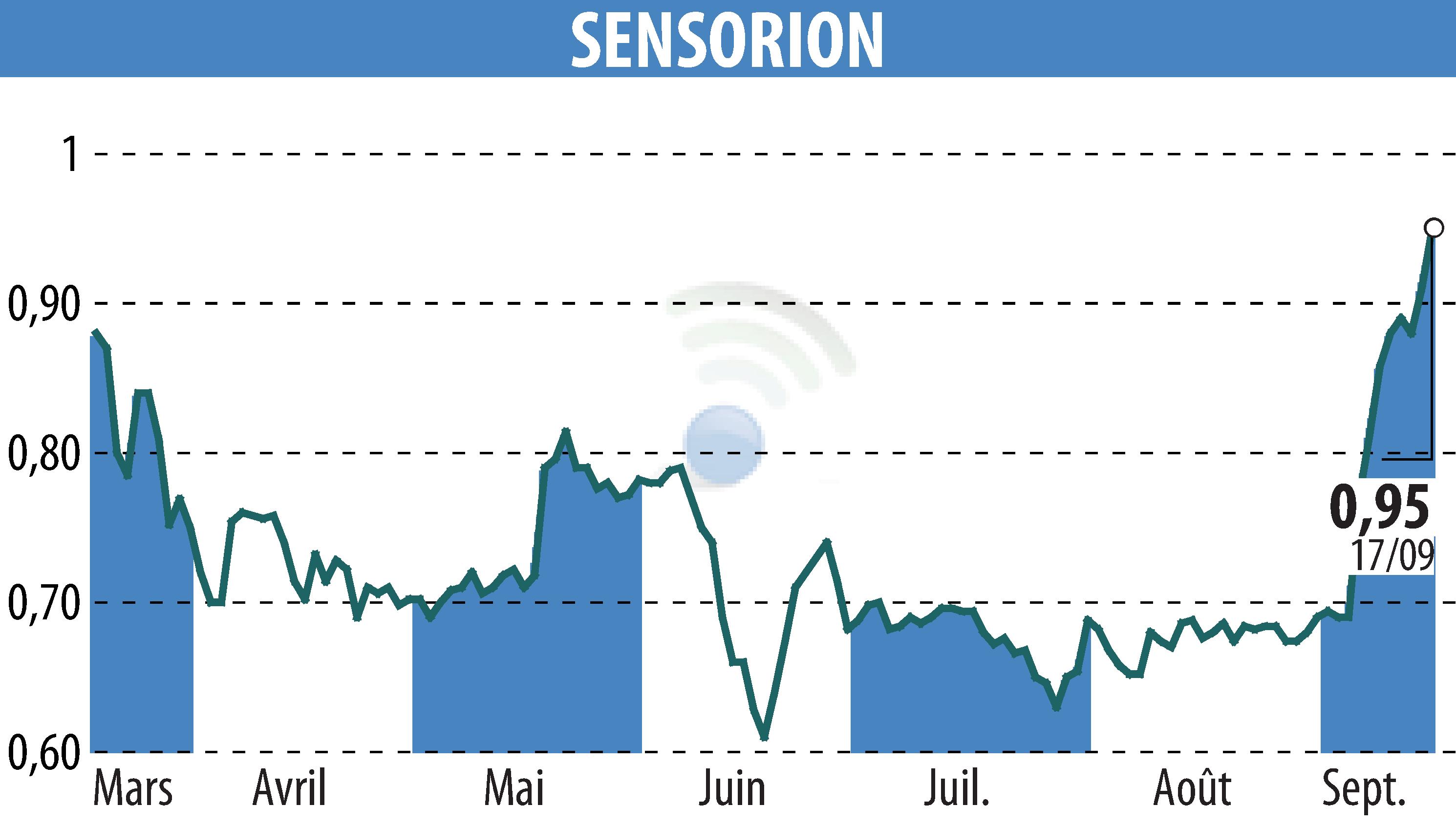
Montpellier, September 18, 2024 – Sensorion, a biotech company specializing in hearing loss therapies, disclosed its financial results for the first half of 2024. The company reported significant progress in its gene therapy and clinical programs.
Sensorion's most advanced program, SENS-501, treated its first patient after receiving European regulatory approval. The preliminary safety data will be presented on September 20, 2024. Additionally, Phase 2a studies for SENS-401 indicated positive efficacy in preventing hearing loss post-cochlear implantation, with full data set for release soon. The company expects to support its operations through to the end of 2025 with a cash reserve of approximately €87 million.
Financial highlights include an 19% increase in R&D expenses to €14.7 million, primarily driven by gene therapy activities, and a net loss of €13.9 million for the period. Sensorion remains focused on advancing its therapies for patients with hereditary and treatment-induced hearing loss.
R. H.
Copyright © 2025 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all SENSORION news
