on MEDINCELL (EPA:MEDCL)
Teva and Medincell Present Promising Results on Olanzapine LAI for Schizophrenia
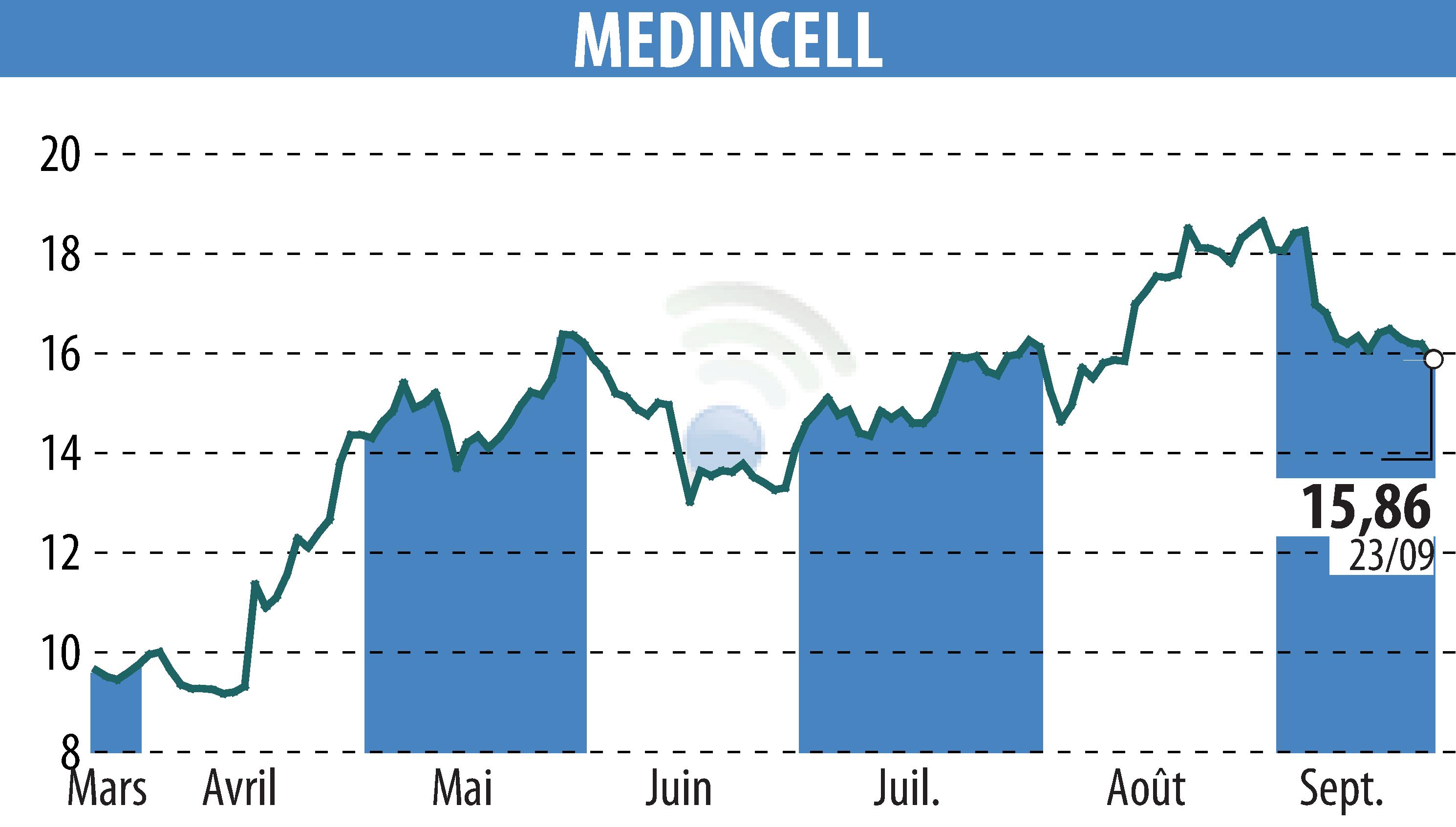
Montpellier, France - Teva, in partnership with Medincell, announced at ENCP 2024 positive results regarding the efficacy, safety and tolerability of the Phase 3 SOLARIS trial of Olanzapine LAI for adult patients with schizophrenia.
The 8-week clinical trial showed that all three tested doses of Olanzapine LAI met their primary and secondary efficacy endpoints, with significant improvements in PANSS, ICG-S and PSP scores compared to placebo. No cases of post-injection sedation syndrome (PDSS) were observed.
Richard Malamut, Medical Director of Medincell, stressed that this new injectable form could facilitate the transition of patients and improve the treatment of schizophrenia thanks to its favorable safety profile.
Teva, which is responsible for the development and commercialization of Olanzapine LAI, could pay Medincell up to $117 million in milestones and royalties in the coming years.
R. P.
Copyright © 2024 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all MEDINCELL news
