on VALNEVA (EPA:VLA)
Valneva SE: Promising Developments in Lyme Disease Vaccine
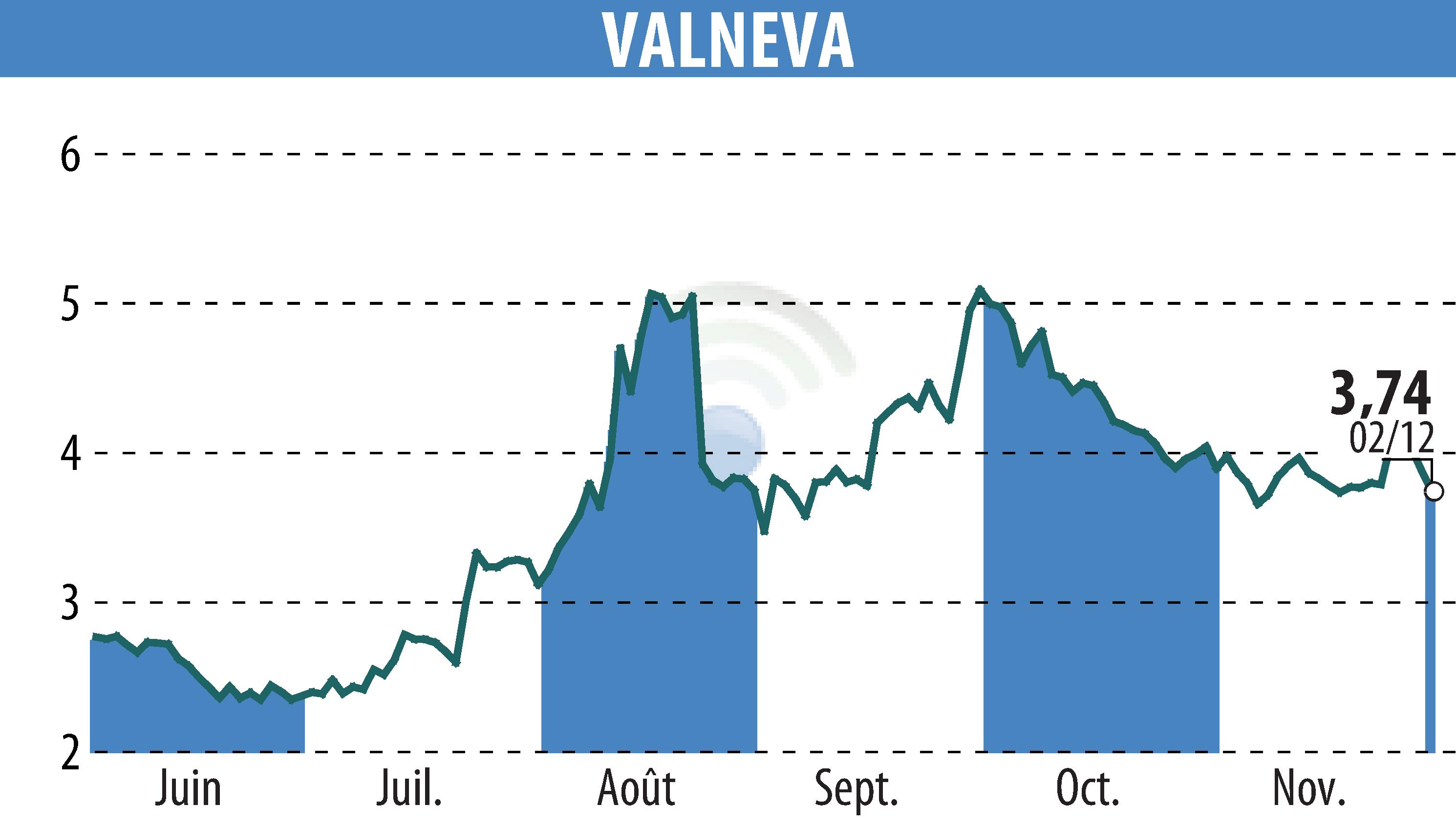
First Berlin Equity Research has reaffirmed its BUY rating for Valneva SE, increasing the target price from €6.30 to €6.80. Valneva, alongside partner Pfizer, is making strides in developing the first human Lyme disease vaccine, VLA15, which has concluded its pivotal phase 3 trial. Trial results are anticipated in the first half of next year, with regulatory approval filings in Europe and the U.S. expected in 2026.
Assuming successful approval, Valneva anticipates launching VLA15 by autumn 2027, potentially generating milestone payments of USD143 million from Pfizer. Beyond this, Valneva could receive an additional USD100 million in commercial milestones and royalties ranging from 14% to 22%.
Valneva and Pfizer view the global Lyme vaccine market as exceeding USD1 billion, though current estimates suggest a potential market value of over USD2 billion, considering the demand in endemic regions.
R. E.
Copyright © 2026 FinanzWire, all reproduction and representation rights reserved.
Disclaimer: although drawn from the best sources, the information and analyzes disseminated by FinanzWire are provided for informational purposes only and in no way constitute an incentive to take a position on the financial markets.
Click here to consult the press release on which this article is based
See all VALNEVA news
